
Out of $5$ isomers of ${C_4}{H_8}$, how many of them are cyclic?
A.$1$
B.$2$
C.$3$
D.$4$
Answer
582.3k+ views
Hint:By the term isomers, we mean two or more compounds with the same formula but a different arrangement of atoms in the molecule and different properties.
Complete step by step answer: In ${C_4}{H_8}$ , there are $4$ carbon atoms and $8$ hydrogen atoms present.
An alkane with $4$ carbon atoms would have the formula ${C_4}{H_{10}}$ . In this, hydrocarbon has two hydrogen less, so it must contain either a double bond or a ring.
We know that, there are isomers of ${C_4}{H_8}$ which are given below:
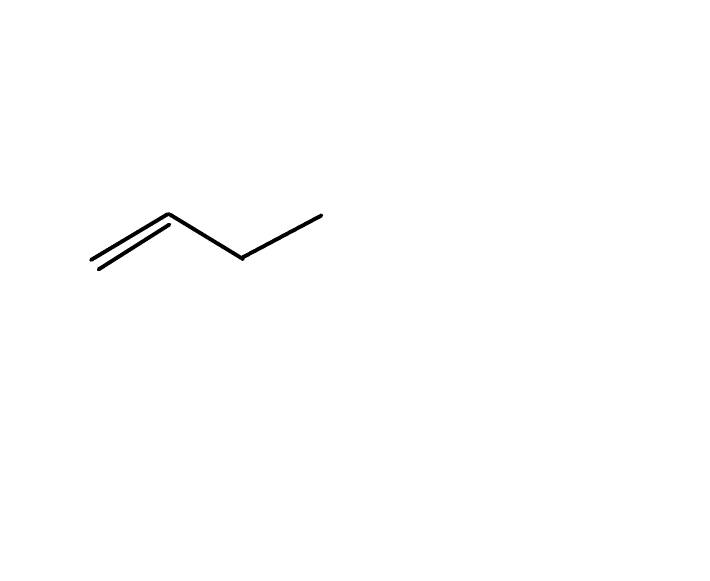
But-$1$-ene
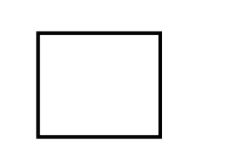
Cyclobutane ( $4$ membered ring with a $C{H_2}$ group at each corner)
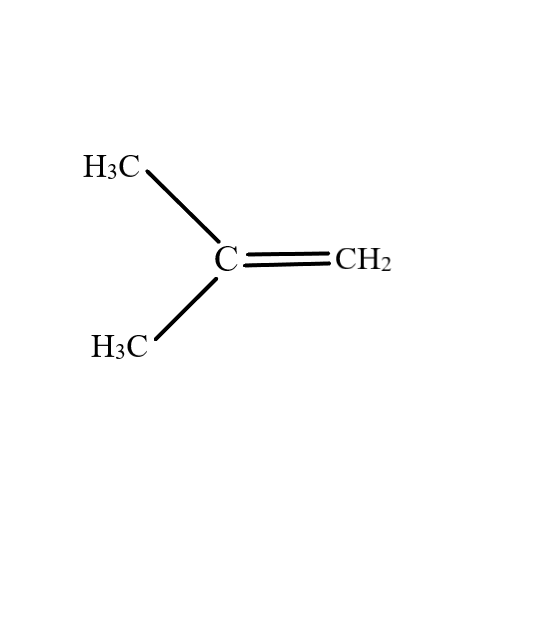
$2$-Methylpropene
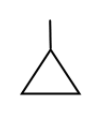
Methylcyclopropane
But-$2$-ene (exists in both cis and trans forms ($2$ geometrical isomers))
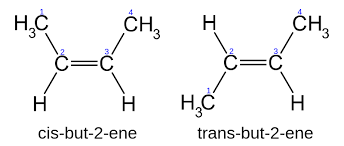
But-$2$-ene can be made into two forms- Trans-$2$-butene and Cis-$2$-butene.
Hence, after studying about these isomers we came to know that there are $2$ cyclic isomers of ${C_4}{H_8}$ i.e. Cyclobutane and Methylcyclopropane.
So, options A, B and D are incorrect.
Therefore, option C is correct i.e. there are $2$ cyclic isomers of ${C_4}{H_8}$.
Note:There are three types of structural isomers: Chain isomers, Functional group isomers and Positional isomers. Isomers which have the same molecular formula but different arrangements or branches are known as Chain Isomers. Isomers that have the same molecular formula but different functional groups are called Functional groups . Positional isomers are structural isomers that can be viewed as differing only on the position of a functional group, substituent, or some other feature on a "parent" structure.
Complete step by step answer: In ${C_4}{H_8}$ , there are $4$ carbon atoms and $8$ hydrogen atoms present.
An alkane with $4$ carbon atoms would have the formula ${C_4}{H_{10}}$ . In this, hydrocarbon has two hydrogen less, so it must contain either a double bond or a ring.
We know that, there are isomers of ${C_4}{H_8}$ which are given below:
But-$1$-ene

Cyclobutane ( $4$ membered ring with a $C{H_2}$ group at each corner)

$2$-Methylpropene

Methylcyclopropane

But-$2$-ene (exists in both cis and trans forms ($2$ geometrical isomers))

But-$2$-ene can be made into two forms- Trans-$2$-butene and Cis-$2$-butene.
Hence, after studying about these isomers we came to know that there are $2$ cyclic isomers of ${C_4}{H_8}$ i.e. Cyclobutane and Methylcyclopropane.
So, options A, B and D are incorrect.
Therefore, option C is correct i.e. there are $2$ cyclic isomers of ${C_4}{H_8}$.
Note:There are three types of structural isomers: Chain isomers, Functional group isomers and Positional isomers. Isomers which have the same molecular formula but different arrangements or branches are known as Chain Isomers. Isomers that have the same molecular formula but different functional groups are called Functional groups . Positional isomers are structural isomers that can be viewed as differing only on the position of a functional group, substituent, or some other feature on a "parent" structure.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




