
Out of benzene and phenol, phenol is more easily nitrated because _________.
A. presence of –OH group in phenol increases the electron density at ortho and para-position
B. presence of –OH group in phenol decreases the electron density at ortho and para-position
C. nitration being electrophilic substitution requires less density at ortho and para position
D. phenol is more reactive than benzene due to R-effect
Answer
592.8k+ views
Hint: This question is based on the resonating structures and the type of group attached to the benzene ring. Either the group is electron donating or the group is electron withdrawing. Nitration is an electrophilic substitution reaction, in which a nitro group is added. The electrophile attacks where there is an electron rich atom.
Complete answer:
Benzene is a six-membered aromatic compound with three double bonds present in it.

Phenol is an alcohol where –OH group is attached to a benzene ring. Let us draw the resonating structures of phenol.
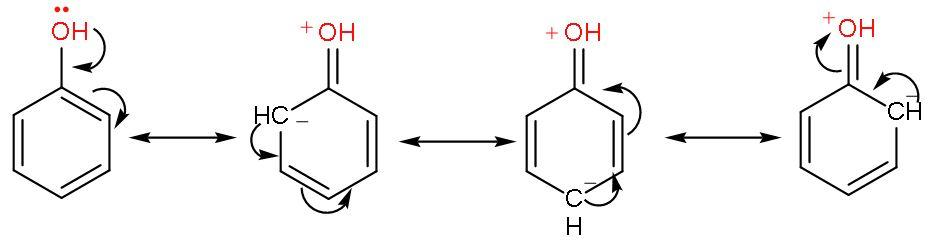
Thus, by resonating structures it is clear that the -OH group attached to the benzene ring is activated. So, it favours electrophilic substitution reactions. Phenol directs the incoming group here nitro; to ‘ortho and para’ positions in the ring as these positions become electron-rich due to presence of negative sign.
But this resonating factor is not present in benzene.
Out of benzene and phenol, phenol gets more easily nitrated because there is -OH group present in phenol which increases the electron density at ortho and para – position. The reaction between phenol and $\text{dil}\text{. HN}{{\text{O}}_{3}}$ is-

Out of benzene and phenol, phenol is more easily nitrated because the presence of –OH group in phenol increases the electron density at ortho and para-position So, the correct answer is “Option A”.
Note: In nitration reaction: Nitrobenzene is formed by the reagents which not only includes but also $\text{conc}\text{. }{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$, $\text{conc}\text{. }{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$ is the source of the nitronium ion which is formed by the protonation of nitric acid by sulphuric acid. This nitronium electrophile reacts with a benzene ring to form nitrobenzene.
Complete answer:
Benzene is a six-membered aromatic compound with three double bonds present in it.

Phenol is an alcohol where –OH group is attached to a benzene ring. Let us draw the resonating structures of phenol.

Thus, by resonating structures it is clear that the -OH group attached to the benzene ring is activated. So, it favours electrophilic substitution reactions. Phenol directs the incoming group here nitro; to ‘ortho and para’ positions in the ring as these positions become electron-rich due to presence of negative sign.
But this resonating factor is not present in benzene.
Out of benzene and phenol, phenol gets more easily nitrated because there is -OH group present in phenol which increases the electron density at ortho and para – position. The reaction between phenol and $\text{dil}\text{. HN}{{\text{O}}_{3}}$ is-

Out of benzene and phenol, phenol is more easily nitrated because the presence of –OH group in phenol increases the electron density at ortho and para-position So, the correct answer is “Option A”.
Note: In nitration reaction: Nitrobenzene is formed by the reagents which not only includes but also $\text{conc}\text{. }{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$, $\text{conc}\text{. }{{\text{H}}_{2}}\text{S}{{\text{O}}_{4}}$ is the source of the nitronium ion which is formed by the protonation of nitric acid by sulphuric acid. This nitronium electrophile reacts with a benzene ring to form nitrobenzene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




