
Out of chlorobenzene and benzyl chloride, which one gets easily hydrolysed by aqueous NaOH and why?
Answer
594.9k+ views
Hint:
Both chlorobenzene and benzyl chloride are organic aromatic compounds and both have a benzene ring. The only difference is that there is one chlorine atom present in chlorobenzene and in benzyl chloride a $C{{H}_{2}}Cl$group is present. The less stable compound among the two will undergo hydrolysis readily.
Complete step by step answer:
As we know the structure benzyl chloride and chlorobenzene is-
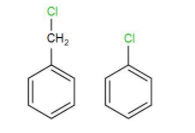
As we can see from their structures, the chlorine atom is directly attached to the benzene ring in chlorobenzene and the carbon-chlorine bond is very stable. Therefore, chlorobenzene has a more stable initial structure compared to benzyl chloride.
Also, due to the presence of the lone pairs on the chlorine atom, it will undergo resonance with the benzene ring to gain extra stability. The resonance structures are-
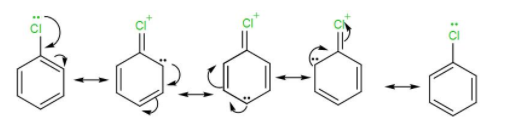
Due to these stability factors, chlorobenzene will not get hydrolysed.
Also, due to the fact that chlorine is highly electronegative and carbon- chlorine bond is extremely strong, it will require a very high energy for the Carbon-chlorine bond to break.
Also, phenol is an electron withdrawing group whereas chlorine is electronegative.
Therefore, it will not undergo substitution to get hydrolysed by NaOH.
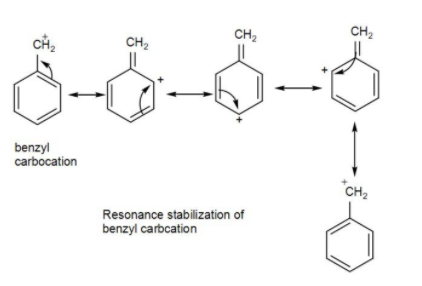
However, in the case of benzyl chloride, as we know it can form a stable intermediate benzyl carbocation, it will have the opportunity to get hydrolysed. The carbocation formed is resonance stabilized-
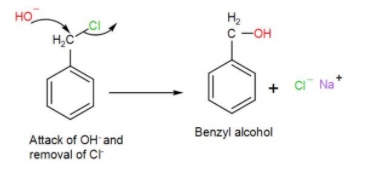
Benzyl chloride undergoes bimolecular nucleophilic substitution,$S{{N}{2}}$ with the formation of a stable transition state and gets hydrolysed forming benzyl alcohol as shown below-
Additional Information:
$S{{N}{2}}$ is a substitution reaction which moves forward via a transition state. Breaking of the leaving group bond and formation of the attacking group bond takes place simultaneously in a single step.
Note:
It is important to remember here that chlorobenzene also undergoes resonance but a stable carbocation is not formed there which is required for the hydrolysis by removal of chlorine and addition of hydroxyl group. Benzyl chloride forms a stable transition state enabling the $S{{N}{2}}$ pathway for hydrolysis forming benzyl alcohol.
Both chlorobenzene and benzyl chloride are organic aromatic compounds and both have a benzene ring. The only difference is that there is one chlorine atom present in chlorobenzene and in benzyl chloride a $C{{H}_{2}}Cl$group is present. The less stable compound among the two will undergo hydrolysis readily.
Complete step by step answer:
As we know the structure benzyl chloride and chlorobenzene is-

As we can see from their structures, the chlorine atom is directly attached to the benzene ring in chlorobenzene and the carbon-chlorine bond is very stable. Therefore, chlorobenzene has a more stable initial structure compared to benzyl chloride.
Also, due to the presence of the lone pairs on the chlorine atom, it will undergo resonance with the benzene ring to gain extra stability. The resonance structures are-

Due to these stability factors, chlorobenzene will not get hydrolysed.
Also, due to the fact that chlorine is highly electronegative and carbon- chlorine bond is extremely strong, it will require a very high energy for the Carbon-chlorine bond to break.
Also, phenol is an electron withdrawing group whereas chlorine is electronegative.
Therefore, it will not undergo substitution to get hydrolysed by NaOH.
However, in the case of benzyl chloride, as we know it can form a stable intermediate benzyl carbocation, it will have the opportunity to get hydrolysed. The carbocation formed is resonance stabilized-

Benzyl chloride undergoes bimolecular nucleophilic substitution,$S{{N}{2}}$ with the formation of a stable transition state and gets hydrolysed forming benzyl alcohol as shown below-

Additional Information:
$S{{N}{2}}$ is a substitution reaction which moves forward via a transition state. Breaking of the leaving group bond and formation of the attacking group bond takes place simultaneously in a single step.
Note:
It is important to remember here that chlorobenzene also undergoes resonance but a stable carbocation is not formed there which is required for the hydrolysis by removal of chlorine and addition of hydroxyl group. Benzyl chloride forms a stable transition state enabling the $S{{N}{2}}$ pathway for hydrolysis forming benzyl alcohol.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




