
Out of (ref. image) which one is more reactive towards nucleophilic substitution reaction and why?
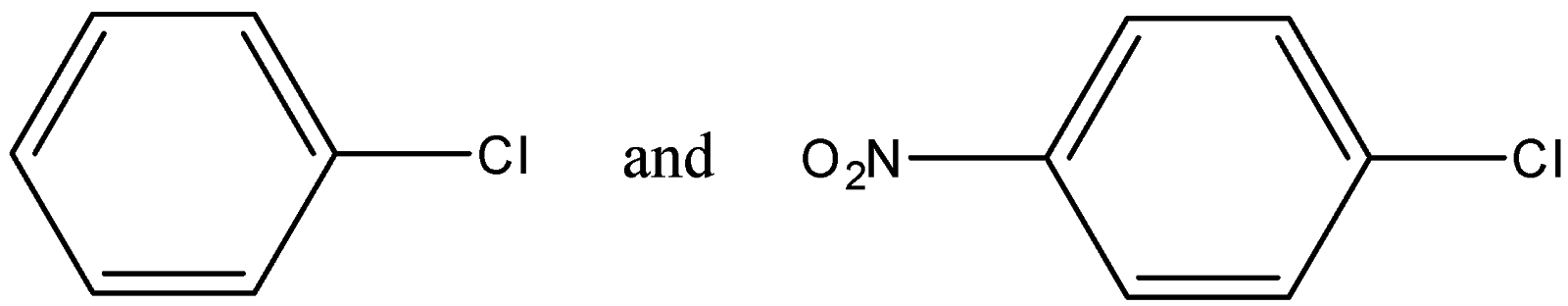
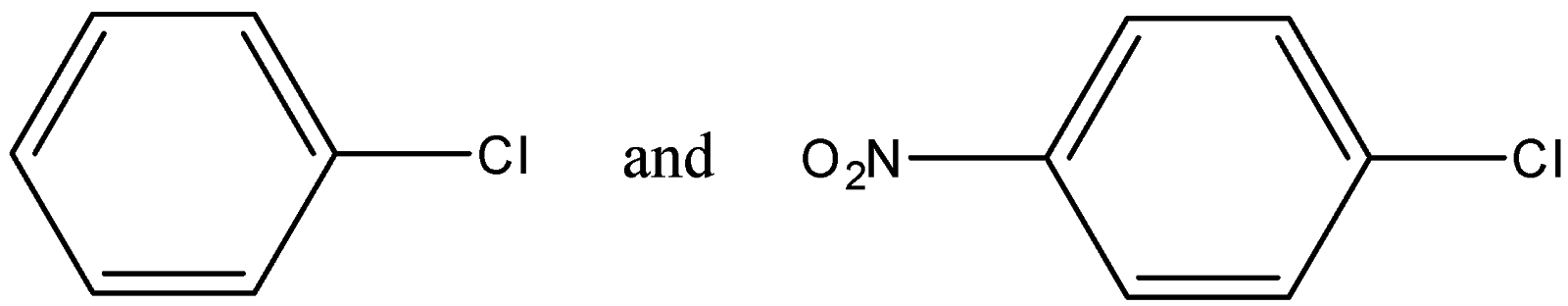
Answer
577.5k+ views
Hint: The nucleophilic substitution reaction is done by the formation of a carbocation and so the positive effects like +M, +I, +H in a compound stabilize it while negative effects –H, -M, -I in a compound destabilize it.
Complete step by step solution:
-There are two types of reagents that attack the carbon centres. One is nucleophile and the other is electrophile. Both are opposite to each other in terms of their behavior.
-Nucleophiles are the substances which are rich in electron density and attack the centre where the electron density is less. They can be negatively charged or neutral with lone pairs of electrons.
-Nucleophilic substitution involves the attack of the nucleophile at the carbon centre which is electron deficient. Usually the attack occurs on the reaction intermediate called carbocation.
-The nucleophile substitutes the leaving group present in the carbon chain and forms the product. The strength of the nucleophile depends on the effects shown by the groups. Positive effects make it stronger while negative effects make it weaker.
-The strength of the positive effects in organic chemistry are arranged in the order as
Mesomeric effect>hyperconjugation>inductive effect
-This order is followed to find the strength of the nucleophilic substitution in any compound. In the given options, 1 compound has a –Cl atom while the other has 2 different atoms at para positions.
-The effect shown by the chloride atom is +H in a benzene ring due to delocalization of the lone pairs. The $N{{O}_{2}}$ group shows the +M effect in a compound which is stronger than +H effect.
-The first compound has only +H effect while the second compound has both +M and +H effects due to the presence of 2 different groups.
Therefore the second one is more reactive towards nucleophilic substitution reaction due to more positive effects in the compound to increase its stability.
Note: The +M effect in any compound does not exist if the group is located at the meta position. The effect is valid only for ortho and para positions. The group usually exhibits the effect of –I at the meta position.
Complete step by step solution:
-There are two types of reagents that attack the carbon centres. One is nucleophile and the other is electrophile. Both are opposite to each other in terms of their behavior.
-Nucleophiles are the substances which are rich in electron density and attack the centre where the electron density is less. They can be negatively charged or neutral with lone pairs of electrons.
-Nucleophilic substitution involves the attack of the nucleophile at the carbon centre which is electron deficient. Usually the attack occurs on the reaction intermediate called carbocation.
-The nucleophile substitutes the leaving group present in the carbon chain and forms the product. The strength of the nucleophile depends on the effects shown by the groups. Positive effects make it stronger while negative effects make it weaker.
-The strength of the positive effects in organic chemistry are arranged in the order as
Mesomeric effect>hyperconjugation>inductive effect
-This order is followed to find the strength of the nucleophilic substitution in any compound. In the given options, 1 compound has a –Cl atom while the other has 2 different atoms at para positions.
-The effect shown by the chloride atom is +H in a benzene ring due to delocalization of the lone pairs. The $N{{O}_{2}}$ group shows the +M effect in a compound which is stronger than +H effect.
-The first compound has only +H effect while the second compound has both +M and +H effects due to the presence of 2 different groups.
Therefore the second one is more reactive towards nucleophilic substitution reaction due to more positive effects in the compound to increase its stability.
Note: The +M effect in any compound does not exist if the group is located at the meta position. The effect is valid only for ortho and para positions. The group usually exhibits the effect of –I at the meta position.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




