
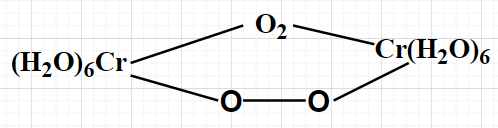
What is the oxidation number of Cr in the following complex?
${\text{A}}{\text{.}}$ 3
${\text{B}}{\text{.}}$ 6
${\text{C}}{\text{.}}$ 4
${\text{D}}{\text{.}}$ 5

Answer
609k+ views
Hint- Here, we will proceed by defining the oxidation number of any atom. Then, we will be using the concept that in a molecule or compound, the oxidation number is the sum of the oxidation numbers of its constituent atoms.
Complete answer:
The oxidation number of an atom is the charge that would exist on the atom if the bonding were completely ionic. In simple ions, the oxidation number of the atom is the charge on the ion. In a molecule or compound, the oxidation number is the sum of the oxidation numbers of its constituent atoms.
Atoms with d-shell electrons can have several different oxidation numbers.
In complex ions or molecules, the oxidation numbers of these atoms can be calculated if we assume that the oxidation numbers of the other atoms in the species are fixed.
The oxidation state of ${\left[ {{\text{Cr}}{{\left( {{{\text{H}}_2}{\text{O}}} \right)}_6}} \right]^{ + 3}}$ is +3 in the given complex compound.
According to the rules for calculating the oxidation states, we have
The oxidation state of O is usually -2.
The oxidation state of H is usually +1.
The sum of all the oxidation states of all the elements present in an ion is equal to the charge on the complete ion.
Using the above concept, oxidation number corresponding to ${{\text{H}}_2}{\text{O}}$ is given by
Since, H = +1, then 2H = +2.
Also, O = -2
Then, 2H + O = +2 - 2 = 0
Thus, the compound ${{\text{H}}_2}{\text{O}}$ do not contribute to the charge on the ion ${\left[ {{\text{Cr}}{{\left( {{{\text{H}}_2}{\text{O}}} \right)}_6}} \right]^{ + 3}}$. This means that the compound ${{\text{H}}_2}{\text{O}}$ is neutral (just like ammonia i.e., ${\text{N}}{{\text{H}}_3}$). The only atom that contributes oxidation number in the ion ${\left[ {{\text{Cr}}{{\left( {{{\text{H}}_2}{\text{O}}} \right)}_6}} \right]^{ + 3}}$ is the Cr atom.
Oxidation number of Cr + 6(Oxidation number of ${{\text{H}}_2}{\text{O}}$) = Charge on the ion ${\left[ {{\text{Cr}}{{\left( {{{\text{H}}_2}{\text{O}}} \right)}_6}} \right]^{ + 3}}$
$ \Rightarrow $Oxidation number of Cr + 6(0) = +3
$ \Rightarrow $Oxidation number of Cr = +3 = 3
Therefore, the required oxidation number of Cr is 3.
Hence, option A is correct.
Note- We can also solve for the oxidation method by directly considering the given complex compound. For this complex compound, 2(Oxidation number of Cr) +4(Oxidation number of O) + 6(Oxidation number of ${{\text{H}}_2}{\text{O}}$) = Charge on the complex compound.
Complete answer:
The oxidation number of an atom is the charge that would exist on the atom if the bonding were completely ionic. In simple ions, the oxidation number of the atom is the charge on the ion. In a molecule or compound, the oxidation number is the sum of the oxidation numbers of its constituent atoms.
Atoms with d-shell electrons can have several different oxidation numbers.
In complex ions or molecules, the oxidation numbers of these atoms can be calculated if we assume that the oxidation numbers of the other atoms in the species are fixed.
The oxidation state of ${\left[ {{\text{Cr}}{{\left( {{{\text{H}}_2}{\text{O}}} \right)}_6}} \right]^{ + 3}}$ is +3 in the given complex compound.
According to the rules for calculating the oxidation states, we have
The oxidation state of O is usually -2.
The oxidation state of H is usually +1.
The sum of all the oxidation states of all the elements present in an ion is equal to the charge on the complete ion.
Using the above concept, oxidation number corresponding to ${{\text{H}}_2}{\text{O}}$ is given by
Since, H = +1, then 2H = +2.
Also, O = -2
Then, 2H + O = +2 - 2 = 0
Thus, the compound ${{\text{H}}_2}{\text{O}}$ do not contribute to the charge on the ion ${\left[ {{\text{Cr}}{{\left( {{{\text{H}}_2}{\text{O}}} \right)}_6}} \right]^{ + 3}}$. This means that the compound ${{\text{H}}_2}{\text{O}}$ is neutral (just like ammonia i.e., ${\text{N}}{{\text{H}}_3}$). The only atom that contributes oxidation number in the ion ${\left[ {{\text{Cr}}{{\left( {{{\text{H}}_2}{\text{O}}} \right)}_6}} \right]^{ + 3}}$ is the Cr atom.
Oxidation number of Cr + 6(Oxidation number of ${{\text{H}}_2}{\text{O}}$) = Charge on the ion ${\left[ {{\text{Cr}}{{\left( {{{\text{H}}_2}{\text{O}}} \right)}_6}} \right]^{ + 3}}$
$ \Rightarrow $Oxidation number of Cr + 6(0) = +3
$ \Rightarrow $Oxidation number of Cr = +3 = 3
Therefore, the required oxidation number of Cr is 3.
Hence, option A is correct.
Note- We can also solve for the oxidation method by directly considering the given complex compound. For this complex compound, 2(Oxidation number of Cr) +4(Oxidation number of O) + 6(Oxidation number of ${{\text{H}}_2}{\text{O}}$) = Charge on the complex compound.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




