
Oxidation number of $S$ in ${H_2}S{O_5}$ is $6$ . This is observed because :
A. There are five oxygen atoms in molecule
B. The hydrogen atom is directly linked with non-metal
C. There is peroxide linkage in the molecule
D. The sulphur atom shows coordinate linkage
Answer
520.1k+ views
Hint: Firstly we have to simplify and mention the process of finding the oxidation state. Then we have to find the oxidation state by the normal state so that we can observe if there is an anomaly or not. If there is any anomaly then we have to mention the structure of the compound so that we can observe what is different and get the answer. In this case the linkage.
Complete step by step answer: There is a basic way to derive the oxidation number for any element in the compound. To do that we just have to apply the pseudo charge due to the electronegativity of the electropositivity of the atom. This is so because all the atoms vary from each other in this aspect. For eg. $F$ is most electronegative so it will always have the $ - 1$ charge.
There are some basic rules in this case:
Hydrogen can have either charge depending on the other atoms.
Metals generally have positive charge
Non-metals generally have negative, and in some cases positive charge, when more negative atoms are present.
Now in this case according to the rules the oxidation number would be :
$
2H + S + 5O = 0 \\
2 + S - 10 = 0 \\
S = 8 \\
$
But in reality the oxidation number is $6$ . This all happens due to the structure of the compound. As oxygen has the peroxy-linkage which reduces the pseudo charge over the atom.
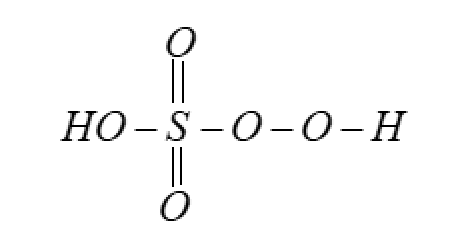
So, the correct answer is “Option C”.
Note: Oxidation number is the pseudo charge on the atom due to being in a set of atoms in a compound. Elements can have different oxidation numbers for the same type of atom in the same compound, like in this case for oxygen. This is because of the variety in bonds.
Complete step by step answer: There is a basic way to derive the oxidation number for any element in the compound. To do that we just have to apply the pseudo charge due to the electronegativity of the electropositivity of the atom. This is so because all the atoms vary from each other in this aspect. For eg. $F$ is most electronegative so it will always have the $ - 1$ charge.
There are some basic rules in this case:
Hydrogen can have either charge depending on the other atoms.
Metals generally have positive charge
Non-metals generally have negative, and in some cases positive charge, when more negative atoms are present.
Now in this case according to the rules the oxidation number would be :
$
2H + S + 5O = 0 \\
2 + S - 10 = 0 \\
S = 8 \\
$
But in reality the oxidation number is $6$ . This all happens due to the structure of the compound. As oxygen has the peroxy-linkage which reduces the pseudo charge over the atom.
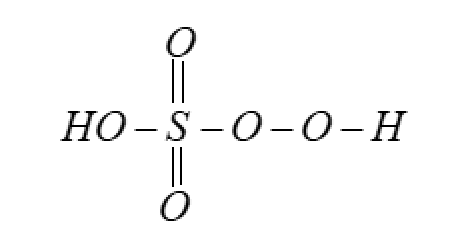
So, the correct answer is “Option C”.
Note: Oxidation number is the pseudo charge on the atom due to being in a set of atoms in a compound. Elements can have different oxidation numbers for the same type of atom in the same compound, like in this case for oxygen. This is because of the variety in bonds.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




