
Oxidation of \[2 - \] methyl propane \[ - 1,2 - \] diol with periodic acid gives
Answer
516.3k+ views
Hint: Periodic acids are also known as Iodic acids. These periodic acids were discovered by the scientist Heinrich Gustav Mangus. Periodic acids or Iodic acids are actually the oxoacids of iodine, in which iodine atoms exist in their highest oxidation states i.e. \[ + 7\] .
Complete answer:
Iodic acid or periodic acid exist in the two forms i.e. as an orthoperiodic acid and as a meta periodic acid.
Let’s know about the chemical formulas of both the forms of the periodic acids.
So, the chemical formula of the Orthoperiodic acid is: \[{H_5}I{O_6}\]
And the chemical formula of the Meta Periodic acid is: \[HI{O_4}\]
In both the forms the oxidation state of the Iodine is \[ + 7\] .
So, let’s proceed with our given chemical equation:
Since, we know that Periodic acids cleaves the diol to give two aldehydic compounds or ketonic compounds.
So, during the oxidation of \[2 - \] methyl propane \[ - 1,2 - \] diol, the diol group is cleaved to yield two carbonyl compounds.
Look at the reaction for a better understanding.
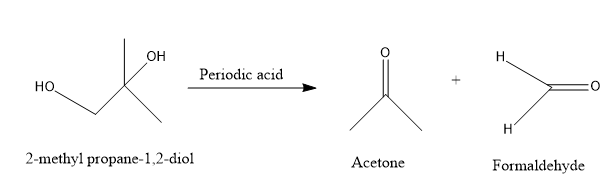
Thus, it is very clear that, during the oxidation of \[2 - \] methyl propane \[ - 1,2 - \] diol, the diol group is cleaved and acetone i.e. a ketonic compound and formaldehyde i.e. an aldehydic compound is produced.
Thus, from the above discussion, the correct answer is option B i.e. Acetone and Formaldehyde.
Note:
Periodic acids are soluble in water and alcohol. They are the strong oxidising agents and generally are used to oxidize glycols. Out of the two forms of periodic acid, the orthoperiodic acid is generally available for commercial use.
Complete answer:
Iodic acid or periodic acid exist in the two forms i.e. as an orthoperiodic acid and as a meta periodic acid.
Let’s know about the chemical formulas of both the forms of the periodic acids.
So, the chemical formula of the Orthoperiodic acid is: \[{H_5}I{O_6}\]
And the chemical formula of the Meta Periodic acid is: \[HI{O_4}\]
In both the forms the oxidation state of the Iodine is \[ + 7\] .
So, let’s proceed with our given chemical equation:
Since, we know that Periodic acids cleaves the diol to give two aldehydic compounds or ketonic compounds.
So, during the oxidation of \[2 - \] methyl propane \[ - 1,2 - \] diol, the diol group is cleaved to yield two carbonyl compounds.
Look at the reaction for a better understanding.

Thus, it is very clear that, during the oxidation of \[2 - \] methyl propane \[ - 1,2 - \] diol, the diol group is cleaved and acetone i.e. a ketonic compound and formaldehyde i.e. an aldehydic compound is produced.
Thus, from the above discussion, the correct answer is option B i.e. Acetone and Formaldehyde.
Note:
Periodic acids are soluble in water and alcohol. They are the strong oxidising agents and generally are used to oxidize glycols. Out of the two forms of periodic acid, the orthoperiodic acid is generally available for commercial use.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




