
Oxidation of naphthalene by acidic \[{\text{KMn}}{{\text{O}}_4}\] gives:
A.Toluene
B.Benzoic acid
C.Benzaldehyde
D.Phthalic acid
Answer
576k+ views
Hint: \[{\text{KMn}}{{\text{O}}_4}\] oxidises double bond. The formation of dicarboxylic acid occurs during the oxidation of naphthalene with KMnO4 in acidic condition.
Complete step by step answer:
When we reacts naphthalene with acidic \[{\text{KMn}}{{\text{O}}_4}\]the oxidation reaction of naphthalene occurs as follow:
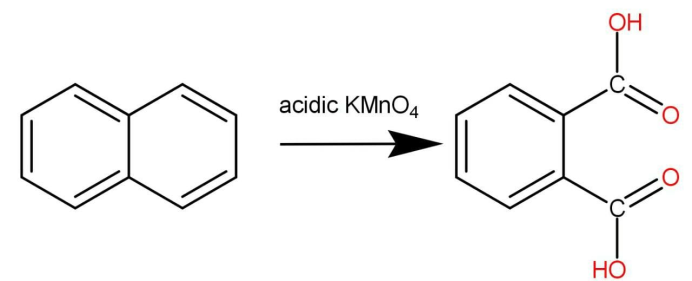
There is one benzene ring and on the other in there is two double bonds the oxidation does not occur on benzene ring because the aromatic ring is highly stable and hence do not react readily so the other ring will react with \[{\text{KMn}}{{\text{O}}_4}\]and two carboxylic acid groups get attached.
The compound formed is known as Phthalic acid. Hence the correct option is D.
Additional information:
Toluene is formed by substitution of one hydrogen from benzene with an alkyl group specifically methyl group. Benzoic acid is a carboxylic acid; the carboxylic acid group is attached with an aromatic benzene ring. Benzaldehyde is an aldehyde on which the aldehyde group is attached with a benzene ring or one hydrogen is substituted with an aldehyde group in benzene.
Potassium permanganate is a strong oxidizing agent and is widely used in laboratories for carrying out volumetric titrations. It is one of the most important oxidizing agents in reagent chemistry. It is deep violet in colour in acidic condition. The oxidation state of manganese in potassium permanganate is 7.
Note:
Naphthalene is an organic compound in which two benzene rings are fused to each other. It is a white crystalline solid. It has a very strong order and can even detect at very low concentration. We usually use naphthalene balls in the household. Naphthalene acts as a fumigant that means it is used to control pests..
Complete step by step answer:
When we reacts naphthalene with acidic \[{\text{KMn}}{{\text{O}}_4}\]the oxidation reaction of naphthalene occurs as follow:

There is one benzene ring and on the other in there is two double bonds the oxidation does not occur on benzene ring because the aromatic ring is highly stable and hence do not react readily so the other ring will react with \[{\text{KMn}}{{\text{O}}_4}\]and two carboxylic acid groups get attached.
The compound formed is known as Phthalic acid. Hence the correct option is D.
Additional information:
Toluene is formed by substitution of one hydrogen from benzene with an alkyl group specifically methyl group. Benzoic acid is a carboxylic acid; the carboxylic acid group is attached with an aromatic benzene ring. Benzaldehyde is an aldehyde on which the aldehyde group is attached with a benzene ring or one hydrogen is substituted with an aldehyde group in benzene.
Potassium permanganate is a strong oxidizing agent and is widely used in laboratories for carrying out volumetric titrations. It is one of the most important oxidizing agents in reagent chemistry. It is deep violet in colour in acidic condition. The oxidation state of manganese in potassium permanganate is 7.
Note:
Naphthalene is an organic compound in which two benzene rings are fused to each other. It is a white crystalline solid. It has a very strong order and can even detect at very low concentration. We usually use naphthalene balls in the household. Naphthalene acts as a fumigant that means it is used to control pests..
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




