
Oxidation state of Fe in $F{{e}_{3}}{{O}_{4}}$ are:
Options are-
(A) + 2 and + 3
(B) + 1 and + 2
(C) + 1 and + 3
(D) None
Answer
570.3k+ views
Hint: An attempt to this question can be made by drawing the expanded structure of the compound. Determine the oxidation state of atoms attached with Fe atoms in the compound. Based on that, determine the oxidation state of iron atoms such that the overall electric charge on the compound cancels out making the compound electrically neutral.
Complete answer:
We will start the solution by understanding the composition of $F{{e}_{3}}{{O}_{4}}$.
$F{{e}_{3}}{{O}_{4}}$ is made up of two oxides of iron namely, Iron(II) oxide and (III) oxide.
We will now draw the expanded structure of both these compounds and determine the oxidation state of iron in these two compounds.
Iron (II) oxide :
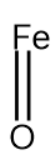
In the structure above we see that the Fe atom is attached to one oxygen atom. The oxidation state of oxygen is -2. So, the oxidation state becomes +2. This is because the total electric charge on the compound should be 0.
Iron (III) oxide :
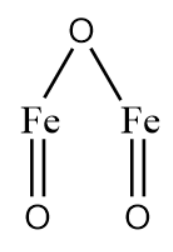
In the structure above we can identify 3 oxygen atoms attached to 2 Fe atoms. The oxidation state of oxygen is -2. Thus, the oxidation state of Fe becomes +3. This is done in order to balance out the charge to make the compound electrically neutral.
Thus, the Oxidation state of Fe in $F{{e}_{3}}{{O}_{4}}$ are:
+ 2 and + 3
The correct answer is option (A).
Note:
It is important to know that the oxidation state of Fe is +3 in Iron(III) oxide and not 6. This is because the -6 charge has to be balanced by 2 Fe atoms. Thus the oxidation state of both the Fe atoms is +3.
Complete answer:
We will start the solution by understanding the composition of $F{{e}_{3}}{{O}_{4}}$.
$F{{e}_{3}}{{O}_{4}}$ is made up of two oxides of iron namely, Iron(II) oxide and (III) oxide.
We will now draw the expanded structure of both these compounds and determine the oxidation state of iron in these two compounds.
Iron (II) oxide :
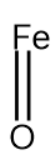
In the structure above we see that the Fe atom is attached to one oxygen atom. The oxidation state of oxygen is -2. So, the oxidation state becomes +2. This is because the total electric charge on the compound should be 0.
Iron (III) oxide :

In the structure above we can identify 3 oxygen atoms attached to 2 Fe atoms. The oxidation state of oxygen is -2. Thus, the oxidation state of Fe becomes +3. This is done in order to balance out the charge to make the compound electrically neutral.
Thus, the Oxidation state of Fe in $F{{e}_{3}}{{O}_{4}}$ are:
+ 2 and + 3
The correct answer is option (A).
Note:
It is important to know that the oxidation state of Fe is +3 in Iron(III) oxide and not 6. This is because the -6 charge has to be balanced by 2 Fe atoms. Thus the oxidation state of both the Fe atoms is +3.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




