
When o-xylene is oxidised with alkaline \[{\text{KMn}}{{\text{O}}_{\text{4}}}\] , the product is:
A) Terephthalic acid
B) Phthalic acid
C) Benzoic acid
D) Salicylic acid
Answer
578.7k+ views
Hint: Alkaline potassium permanganate oxidizes benzene side chain. The die chain of benzene is an aliphatic group. Irrespective of the number of the carbon atoms in the benzene size chain, it is oxidized to aromatic carboxylic groups.
Complete answer:
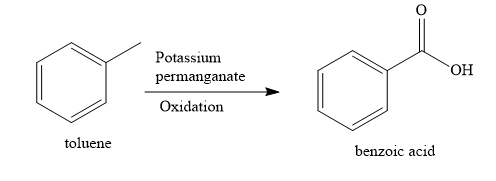
When toluene (methyl benzene) is oxidized with alkaline potassium permanganate solution, benzoic acid product is obtained. The aliphatic methyl group is oxidized to the aromatic carboxylic functional group.
Write the structure of o-xylene
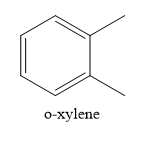
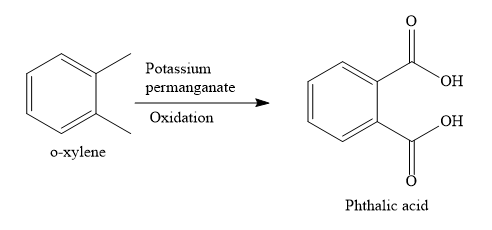
o-xylene is a disubstituted benzene, Two, methyl groups are present on 1,2 positions.
When o-xylene is oxidized with potassium permanganate solution, both the methyl groups are oxidized to carboxylic groups. None of the aromatic carbon atoms undergo oxidation reaction. Only aliphatic carbon atoms are oxidized.
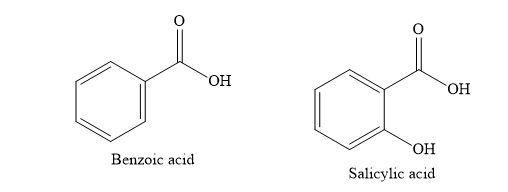
Write the structures of various compounds given in the options.
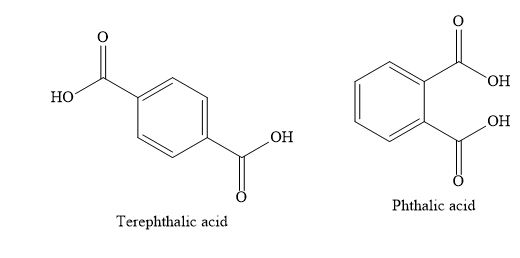
When o-xylene is oxidized with potassium permanganate solution, the product obtained is phthalic acid.
Additional Information: Potassium permanganate is a strong oxidizing agent. It oxidizes alcohols, aldehydes and ketones. The end product in these oxidations is a carboxylic acid.
Note: Thus, the oxidation of methyl benzene, ethyl benzene, propyl benzene and cumene with alkaline potassium permanganate solution will give only one product benzoic acid. This is because the entire alkyl side chain is oxidized to an aromatic carboxylic group.
Complete answer:
When toluene (methyl benzene) is oxidized with alkaline potassium permanganate solution, benzoic acid product is obtained. The aliphatic methyl group is oxidized to the aromatic carboxylic functional group.

Write the structure of o-xylene

o-xylene is a disubstituted benzene, Two, methyl groups are present on 1,2 positions.
When o-xylene is oxidized with potassium permanganate solution, both the methyl groups are oxidized to carboxylic groups. None of the aromatic carbon atoms undergo oxidation reaction. Only aliphatic carbon atoms are oxidized.
Write the structures of various compounds given in the options.


When o-xylene is oxidized with potassium permanganate solution, the product obtained is phthalic acid.

Additional Information: Potassium permanganate is a strong oxidizing agent. It oxidizes alcohols, aldehydes and ketones. The end product in these oxidations is a carboxylic acid.
Note: Thus, the oxidation of methyl benzene, ethyl benzene, propyl benzene and cumene with alkaline potassium permanganate solution will give only one product benzoic acid. This is because the entire alkyl side chain is oxidized to an aromatic carboxylic group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




