
How ozone was formed first of all?
Answer
584.1k+ views
Hint: The ozone layer is formed by the process known as Chapman cycle and if this layer wasn’t present the DNA would have been damaged which suppresses the immune system resulting in an increase in an infectious disease like skin cancer, eye, cataracts.
Complete step by step answer:
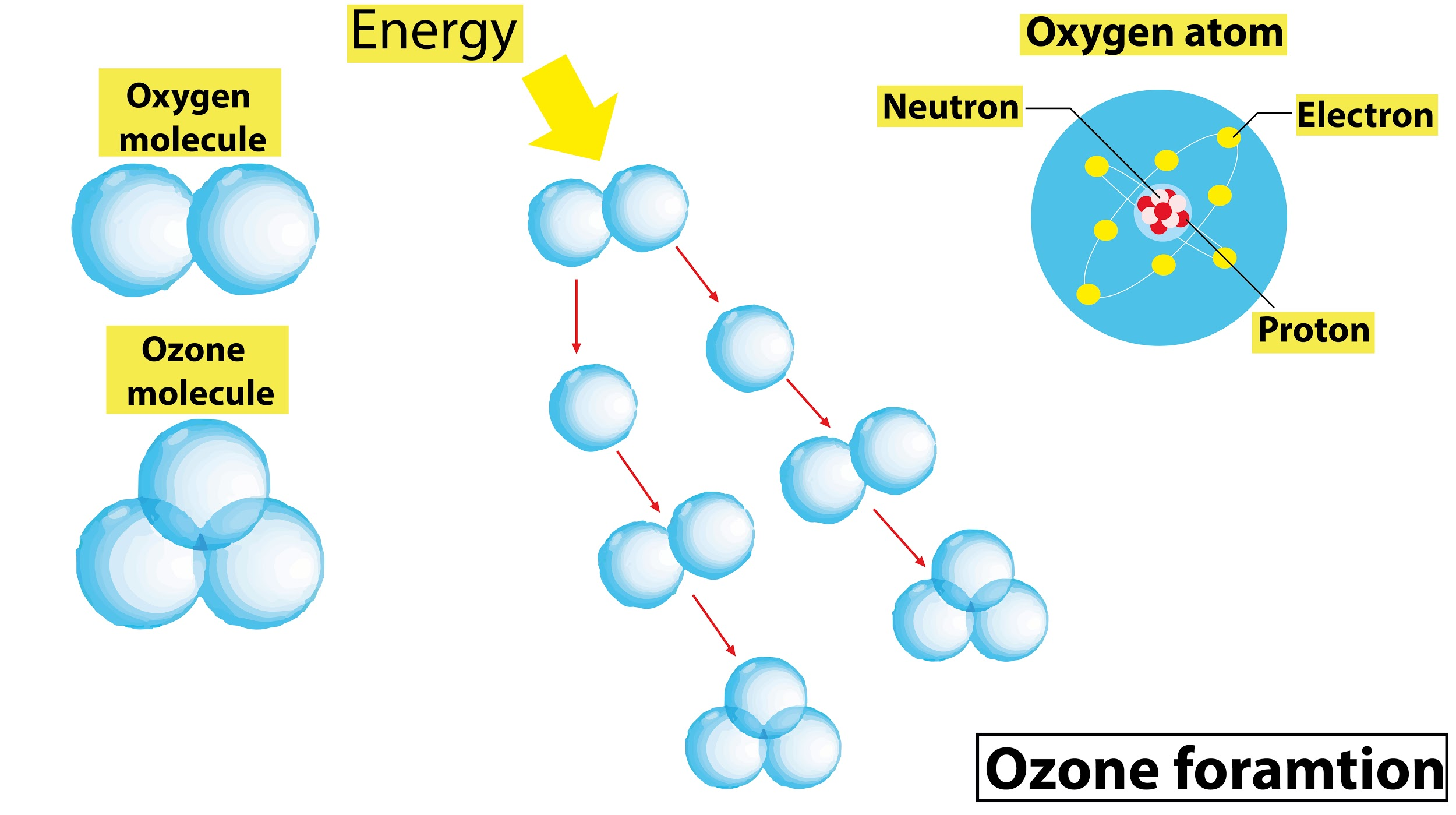
In the Chapman cycle, the oxygen gets photolyzed by U.V rays to form oxygen radicals. Two oxygen molecules are formed and the chlorine atom is freed to destroy ozone.
The formation of the ozone layer:
- The formation of the ozone in the stratosphere region starts with the photodissociation of oxygen molecules by the solar radiation at wavelengths below 240 nm.
\[{{O}_{2}}\xrightarrow{UV<240nm}O+O\]
- The reactive oxygen atoms which are high combine with oxygen molecules to form ozone as follows :
$O+{{O}_{2}}+M\to {{O}_{3}}+M$
M: Some inert substance.
- The role of M in this reaction is to absorb some of the remaining energy released and prevent the spontaneous decomposition of the ${{O}_{3}}$.
\[{{O}_{3}}\xrightarrow{UV}O+{{O}_{2}}\]
Additional Information:
Ozone Depletion:
- Gases from industries and our daily life like chlorofluorocarbons (CFCs), halons, halogens, etc. get involved in the atmosphere which are the main cause for ozone depletion, as it contains or produces chlorine atoms.
Process of Ozone Depletion:
Step I-The U.V. radiation reacts with the CFC molecules stripping of a chlorine atom.
Step II-The Cl atom strikes an oxygen molecule resulting in an oxygen molecule and a chlorine monoxide molecule.
- A free atom of oxygen collides with choline monoxide.
Causes of Ozone Depletion:
- The pollutants that most adversely affect the ozone layer depletion are fluorocarbons.
- Most of the depletion of the ozone layer has been attributed to pollutants containing chlorine from CFCs.
- Chlorofluorocarbons were used in refrigeration and air conditioning systems and as propellants in spray cones. These chemicals act as a catalyst in a chemical reaction that converts ozone to oxygen.
- CFCs are not consumed in the reaction but remain in the stratosphere to continue to cause destruction of the ozone.
Note: Effects of Ozone Depletion:
- Due to ozone depletion harmful U.V. rays such as UV- B radiation reaches to earth on animals, plants, aquatic life as well as on humans also.
- Effect on the aquatic system by affecting phytoplankton, fish, larval carb. The decrease in the amount of phytoplankton increases the carbon dioxide in the atmosphere which contributes to global warming.
- Effect on material by the degradation of paints and plastic.
- Effect on climate by global warming which increases the average temperature of the earth’s surface.
- Ground-level somg increase in the formation of ground- level ozone as a pollutant.
Complete step by step answer:
In the Chapman cycle, the oxygen gets photolyzed by U.V rays to form oxygen radicals. Two oxygen molecules are formed and the chlorine atom is freed to destroy ozone.
The formation of the ozone layer:
- The formation of the ozone in the stratosphere region starts with the photodissociation of oxygen molecules by the solar radiation at wavelengths below 240 nm.
\[{{O}_{2}}\xrightarrow{UV<240nm}O+O\]
- The reactive oxygen atoms which are high combine with oxygen molecules to form ozone as follows :
$O+{{O}_{2}}+M\to {{O}_{3}}+M$
M: Some inert substance.
- The role of M in this reaction is to absorb some of the remaining energy released and prevent the spontaneous decomposition of the ${{O}_{3}}$.
\[{{O}_{3}}\xrightarrow{UV}O+{{O}_{2}}\]

Additional Information:
Ozone Depletion:
- Gases from industries and our daily life like chlorofluorocarbons (CFCs), halons, halogens, etc. get involved in the atmosphere which are the main cause for ozone depletion, as it contains or produces chlorine atoms.
Process of Ozone Depletion:
Step I-The U.V. radiation reacts with the CFC molecules stripping of a chlorine atom.
Step II-The Cl atom strikes an oxygen molecule resulting in an oxygen molecule and a chlorine monoxide molecule.
- A free atom of oxygen collides with choline monoxide.
Causes of Ozone Depletion:
- The pollutants that most adversely affect the ozone layer depletion are fluorocarbons.
- Most of the depletion of the ozone layer has been attributed to pollutants containing chlorine from CFCs.
- Chlorofluorocarbons were used in refrigeration and air conditioning systems and as propellants in spray cones. These chemicals act as a catalyst in a chemical reaction that converts ozone to oxygen.
- CFCs are not consumed in the reaction but remain in the stratosphere to continue to cause destruction of the ozone.
Note: Effects of Ozone Depletion:
- Due to ozone depletion harmful U.V. rays such as UV- B radiation reaches to earth on animals, plants, aquatic life as well as on humans also.
- Effect on the aquatic system by affecting phytoplankton, fish, larval carb. The decrease in the amount of phytoplankton increases the carbon dioxide in the atmosphere which contributes to global warming.
- Effect on material by the degradation of paints and plastic.
- Effect on climate by global warming which increases the average temperature of the earth’s surface.
- Ground-level somg increase in the formation of ground- level ozone as a pollutant.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




