
Ozonolysis products of 2-methyl-2-butene are:
A) 2 moles of \[HCHO\]
B) acetaldehyde + formaldehyde
C) acetaldehyde and acetone
D) acetone and formaldehyde
Answer
563.4k+ views
Hint: The product of the Ozonolysis reaction is aldehyde or ketones or a mixture of aldehyde and ketone. Ozonolysis reaction indicates the addition of the ozone molecule across multiple bonds that is the double or triple bond of the reactant species. Symmetrical alkenes give 2 moles of either same product molecules, while unsymmetrical alkenes give two moles of different molecules.
Complete solution:
Ozonolysis reaction indicates the addition of the ozone molecule along with the double bond. In the first step of the reaction ozone molecule is added across the double bond of the alkene 2-methyl-2-butene leads to form the ozonide as shows in the mechanism given below.In the ozonolysis reaction, both the pi bond as well as the sigma bond of the organic compound is broken down.
Here, the mechanism for the Ozonolysis reaction of 2-methyl-2-butene which is an asymmetric alkene as follows:
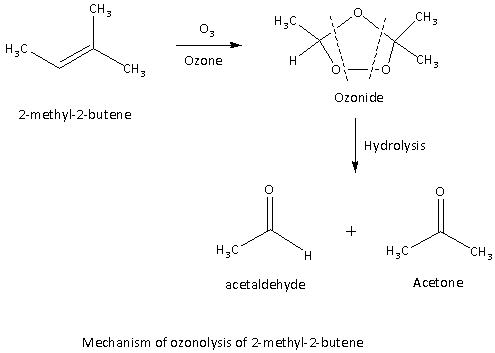
Here, we can see that the ozonide formed upon hydrolysis gives the carbonyl compounds that is one mole of acetaldehyde and one mole of acetone.
Hence, the option (C) is the correct answer to the question.
Note: In the ozonolysis reaction the compounds containing a double bond, triple bonds are converted into carbonyl compounds. Where ozone molecule acts as an oxidizing agent causes oxidation of unsaturated compounds.The product of ozonolysis reaction is always either 2 moles of aldehyde or 2 moles of ketone or one mole of aldehyde and one mole of the ketone.
Complete solution:
Ozonolysis reaction indicates the addition of the ozone molecule along with the double bond. In the first step of the reaction ozone molecule is added across the double bond of the alkene 2-methyl-2-butene leads to form the ozonide as shows in the mechanism given below.In the ozonolysis reaction, both the pi bond as well as the sigma bond of the organic compound is broken down.
Here, the mechanism for the Ozonolysis reaction of 2-methyl-2-butene which is an asymmetric alkene as follows:

Here, we can see that the ozonide formed upon hydrolysis gives the carbonyl compounds that is one mole of acetaldehyde and one mole of acetone.
Hence, the option (C) is the correct answer to the question.
Note: In the ozonolysis reaction the compounds containing a double bond, triple bonds are converted into carbonyl compounds. Where ozone molecule acts as an oxidizing agent causes oxidation of unsaturated compounds.The product of ozonolysis reaction is always either 2 moles of aldehyde or 2 moles of ketone or one mole of aldehyde and one mole of the ketone.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




