
P in $PC{{l}_{5}}$ has $s{{p}^{3}}d$ hybridization which of the following statements is wrong about structure?
A. two P-Cl bonds are stronger and three P-Cl bonds are weaker
B. two P-Cl bonds are axial and larger than three P-Cl equatorial bonds.
C. $PC{{l}_{5}}$has trigonal bipyramidal geometry with non-polar nature
D. All of these
Answer
565.5k+ views
Hint: To solve this question, we should use the VSEPR theory, according to this theory we can find the shape from hybridisation. In $PC{{l}_{5}}$ there are two types of P-Cl bonds present, that are axial and equatorial. The longer bond is weaker and the shorter among them is the stronger bond.
Company answer:
- In the first option it is said that two P-Cl bonds are stronger and three P-Cl bonds are weaker. As we know that there are 3 equatorial bonds and 2 axial bonds and the axial bonds are basically longer. Let’s draw the structure of $PC{{l}_{5}}$:
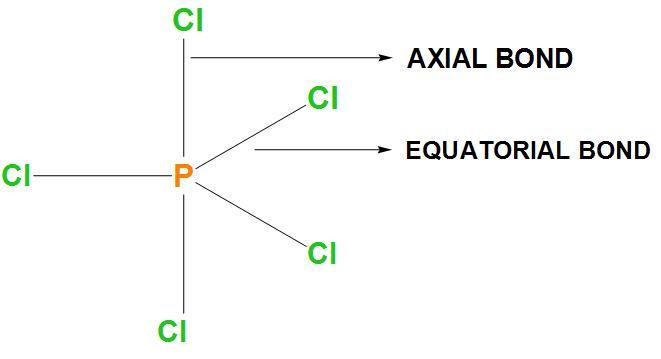
- It is found that bond strength is inversely proportional to the bond length. Hence, the longer bond is not stronger. Therefore, we can say that this statement is incorrect.
Hence, we can conclude that the correct option is (a), that is the statement which is wrong about structure of $PC{{l}_{5}}$ is: two P-Cl bonds are stronger and three P-Cl bonds are weaker.
Note:
- It is found that $PC{{l}_{5}}$ is a chemical compound that is mainly used as a chlorinating reagent.it is found that $PC{{l}_{5}}$ converts alcohols to alkyl chlorides and carboxylic acids to acyl chlorides. But its structure depends on the environment, in the gaseous and molten state it has trigonal bipyramidal geometry and in solid state it becomes ionic compound, $PCl_{4}^{+},PCl_{6}^{-}$
Company answer:
- In the first option it is said that two P-Cl bonds are stronger and three P-Cl bonds are weaker. As we know that there are 3 equatorial bonds and 2 axial bonds and the axial bonds are basically longer. Let’s draw the structure of $PC{{l}_{5}}$:

- It is found that bond strength is inversely proportional to the bond length. Hence, the longer bond is not stronger. Therefore, we can say that this statement is incorrect.
Hence, we can conclude that the correct option is (a), that is the statement which is wrong about structure of $PC{{l}_{5}}$ is: two P-Cl bonds are stronger and three P-Cl bonds are weaker.
Note:
- It is found that $PC{{l}_{5}}$ is a chemical compound that is mainly used as a chlorinating reagent.it is found that $PC{{l}_{5}}$ converts alcohols to alkyl chlorides and carboxylic acids to acyl chlorides. But its structure depends on the environment, in the gaseous and molten state it has trigonal bipyramidal geometry and in solid state it becomes ionic compound, $PCl_{4}^{+},PCl_{6}^{-}$
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




