
p-amino azo benzene is obtained by treating diazonium chloride with:
A. Phenol
B. Benzoic acid
C. Alcohol
D. Aniline
Answer
536.1k+ views
Hint: The given product name is p-amino azo benzene. The structure of the p-amino azo benzene and the structure of diazonium chloride are as follows.
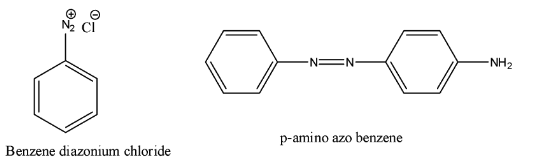
Complete step-by-step answer:- In the question it is given that to find the chemical name which is going to react with diazonium chloride to give p-amino azo benzene.
- First we should know the structure of the p-amino azo benzene and the structure of diazonium chloride.
- The structure of p-amino azo benzene and the structure of diazonium chloride are as follows.
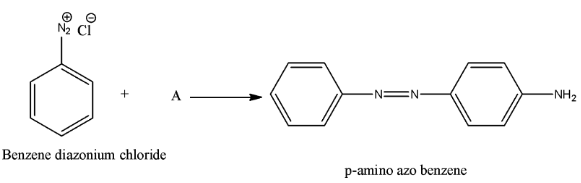
- We have to find the chemical A in the below equation.
- Coming to the options, option A. The chemical is A. Phenol. If we will take we get p-hydroxy azobenzene as the product and the chemical reaction is as follows.
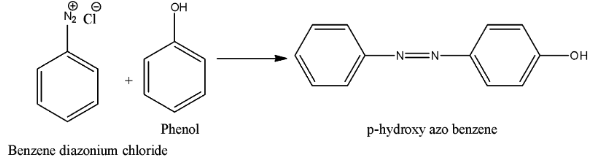
- Therefore we are not getting p-amino azo benzene if we will take A is phenol. So option A is wrong.
- Coming to option B, Benzoic acid. The chemical reaction is as follows.
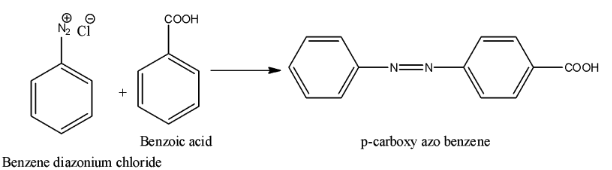
- Therefore option B is also not correct because we didn’t get the product that we need.
- Coming to option C, Alcohol. The chemical reaction is as follows.
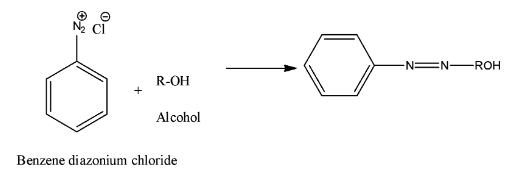
- Therefore the option C is also wrong.
- Coming to option D, Aniline. The chemical reaction is as follows.
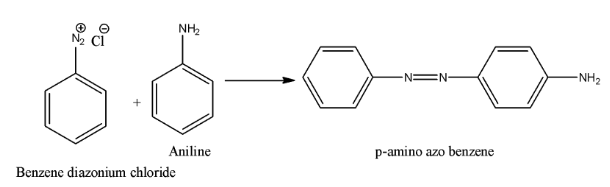
Therefore the option D, aniline is correct.
Note:By using diazonium chloride we can prepare various types of products. For example by using diazonium chloride we can prepare p-amino azo benzene as the product. It is an azo dye useful for various purposes.

Complete step-by-step answer:- In the question it is given that to find the chemical name which is going to react with diazonium chloride to give p-amino azo benzene.
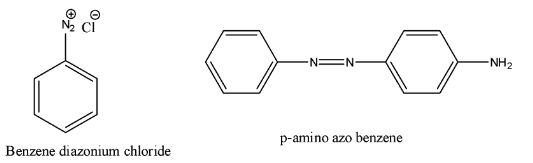
- First we should know the structure of the p-amino azo benzene and the structure of diazonium chloride.
- The structure of p-amino azo benzene and the structure of diazonium chloride are as follows.

- We have to find the chemical A in the below equation.

- Coming to the options, option A. The chemical is A. Phenol. If we will take we get p-hydroxy azobenzene as the product and the chemical reaction is as follows.

- Therefore we are not getting p-amino azo benzene if we will take A is phenol. So option A is wrong.
- Coming to option B, Benzoic acid. The chemical reaction is as follows.

- Therefore option B is also not correct because we didn’t get the product that we need.
- Coming to option C, Alcohol. The chemical reaction is as follows.

- Therefore the option C is also wrong.
- Coming to option D, Aniline. The chemical reaction is as follows.

Therefore the option D, aniline is correct.
Note:By using diazonium chloride we can prepare various types of products. For example by using diazonium chloride we can prepare p-amino azo benzene as the product. It is an azo dye useful for various purposes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




