
$P{{F}_{5}}$ is:
[A] Monophorofluoride
[B] Phosphorus pentafluoride
[C] Pentaphosphoro fluoride
[D] Phosphorus tetrafluoride
[E] Potassium pentafluoride
Answer
589.2k+ views
HINT: The chemical name of P is phosphorus and that of F is fluorine. In the given formula, we have 1 atom of surrounded which is bonded to 5 atoms of fluorine. To find its name, use the IUPAC prefix used to denote the number of an element in the given compound.
COMPLETE STEP BY STEP SOLUTION:
The compound given to us is$P{{F}_{5}}$. It is a colourless, toxic gas and is a halide of phosphorus. We know that the chemical symbol of phosphorus is ‘P’ and that of fluorine is ‘F’, which is a halide. Therefore, we can say that the given gas is a phosphorus-fluorine based gas.
Now let us go through the options to find out its name.
In the first option we have monophorofluoride. The gas given to us has one phosphorous and one phosphorus atom but 5 fluorine atoms. Therefore it cannot have a ‘mono’ prefix and then the full name. Therefore, this is not the correct answer.
Then we have phosphorus pentafluoride. The formula given to us has 1 atom of phosphorus and 5 atoms of fluorine. The prefix –penta can be used for these 5 atoms and for the single phosphorus atom there is no need to add any prefix. Therefore, this can be the correct answer.
Then we have pentaphosphorus fluoride. In this option it says 5 atoms of phosphorus but this is not the case in the formula that is given to us. Therefore, this is not the correct answer.
Next we have phosphorus tetrafluoride. The prefix –tetra is used for 4 atoms. Here, we have 5 fluorine atoms. Therefore, this is not the correct answer.
And lastly, we have potassium pentafluoride. Here, we have potassium whose chemical symbol is ‘K’. Therefore, the formula $P{{F}_{5}}$ cannot have the name potassium pentafluoride.
We can see from the above discussion that phosphorus pentafluoride is the name of $P{{F}_{5}}$.
Therefore, the correct answer is option [B] phosphorus pentafluoride.
NOTE: Phosphorus pentafluoride has trigonal bipyramidal geometry. It has two axial P-F bonds and three equatorial P-F bonds. The length of the axial and the equatorial bond is different in the solid phase but not in the liquid and gaseous state. It is also a Lewis acid and thus ready for hydrolysis.
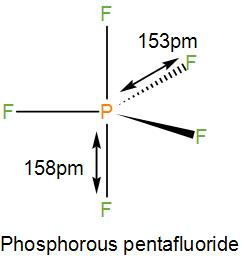
COMPLETE STEP BY STEP SOLUTION:
The compound given to us is$P{{F}_{5}}$. It is a colourless, toxic gas and is a halide of phosphorus. We know that the chemical symbol of phosphorus is ‘P’ and that of fluorine is ‘F’, which is a halide. Therefore, we can say that the given gas is a phosphorus-fluorine based gas.
Now let us go through the options to find out its name.
In the first option we have monophorofluoride. The gas given to us has one phosphorous and one phosphorus atom but 5 fluorine atoms. Therefore it cannot have a ‘mono’ prefix and then the full name. Therefore, this is not the correct answer.
Then we have phosphorus pentafluoride. The formula given to us has 1 atom of phosphorus and 5 atoms of fluorine. The prefix –penta can be used for these 5 atoms and for the single phosphorus atom there is no need to add any prefix. Therefore, this can be the correct answer.
Then we have pentaphosphorus fluoride. In this option it says 5 atoms of phosphorus but this is not the case in the formula that is given to us. Therefore, this is not the correct answer.
Next we have phosphorus tetrafluoride. The prefix –tetra is used for 4 atoms. Here, we have 5 fluorine atoms. Therefore, this is not the correct answer.
And lastly, we have potassium pentafluoride. Here, we have potassium whose chemical symbol is ‘K’. Therefore, the formula $P{{F}_{5}}$ cannot have the name potassium pentafluoride.
We can see from the above discussion that phosphorus pentafluoride is the name of $P{{F}_{5}}$.
Therefore, the correct answer is option [B] phosphorus pentafluoride.
NOTE: Phosphorus pentafluoride has trigonal bipyramidal geometry. It has two axial P-F bonds and three equatorial P-F bonds. The length of the axial and the equatorial bond is different in the solid phase but not in the liquid and gaseous state. It is also a Lewis acid and thus ready for hydrolysis.

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




