
$ Phenol $ , $ {C_6}{H_5}OH $ , is allowed to react with $ diazomethane $ , $ C{H_2}{N_2} $ . The product formed is
(A) $ {C_6}{H_5}C{H_2}OH $
(B) $ {C_6}{H_5}OC{H_3} $
(C)
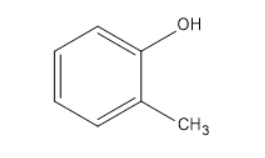
(D)
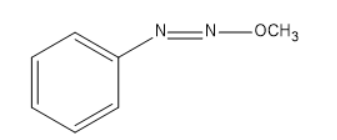


Answer
510.3k+ views
Hint: $ Diazomethane $ is a very reactive compound. It acts as a methylating agent. $ Diazomethane $ converts alcohol into ethers in the presence of ether. To solve this question remember the fact that $ Diazomethane $ is an alkylating agent therefore alkylates the phenol.
Complete answer:
$ Phenol $ is an aromatic organic compound which is a volatile compound. $ Phenol $ has a widespread use involving as a disinfectant, mouthwash, medicinal use like that in surgical antiseptics. $ Phenol $ are similar to alcohols but they form stronger hydrogen bonds that is why they are more soluble in water than are alcohols. $ Phenol $ was first mined from coal tar, but today it is manufactured on a large scale from petroleum. It is an important industrial product as a pioneer to various materials and useful compounds. $ Phenol $ and its chemical products are very important for the production of Bakelite, polycarbonates, detergents, nylon, epoxies, herbicides such as phenoxy herbicides.
$ Diazomethane $ is the simplest diazo compound. As mentioned in the hint $ Diazomethane $ converts alcohol into ethers. This is due to the fact that $ - C{H_2} $ group attaches to the alcohol group releasing molecular nitrogen.
$ Diazomethane $ when reacting with a $ Phenol $ acts as a base and abstracts a proton from phenol. This results in the formation of $ phenoxide $ ions. After this $ phenoxide $ ion acts as a nucleophile and under this nucleophilic attack $ Anisole $ ether is formed.
The reaction is shown below:
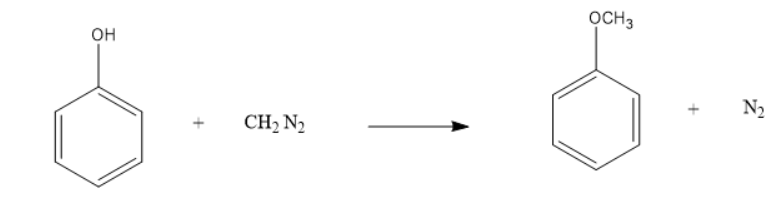
$ Anisole $ is formed in the reaction and nitrogen gas is liberated when $ Diazomethane $ reacts with . $ Phenol $ . This reaction takes place in the presence of $ Fluoroboric $ $ acid $ .
$ Anisole $ or $ methoxybenzene $ is an organic compound which is a colourless liquid. Its smell is reminiscent of anise seed. Due to this smell it is used in making perfumes, flavouring agents as well as solvent.
Note:
We not only use $ Fluoroboric $ $ acid $ to enhance the rate of reaction but also chemicals like $ Sodium{\text{ }}hydroxide $ which increases the rate of reaction. During such reactions, liberation of nitrogen always takes place.
Complete answer:
$ Phenol $ is an aromatic organic compound which is a volatile compound. $ Phenol $ has a widespread use involving as a disinfectant, mouthwash, medicinal use like that in surgical antiseptics. $ Phenol $ are similar to alcohols but they form stronger hydrogen bonds that is why they are more soluble in water than are alcohols. $ Phenol $ was first mined from coal tar, but today it is manufactured on a large scale from petroleum. It is an important industrial product as a pioneer to various materials and useful compounds. $ Phenol $ and its chemical products are very important for the production of Bakelite, polycarbonates, detergents, nylon, epoxies, herbicides such as phenoxy herbicides.
$ Diazomethane $ is the simplest diazo compound. As mentioned in the hint $ Diazomethane $ converts alcohol into ethers. This is due to the fact that $ - C{H_2} $ group attaches to the alcohol group releasing molecular nitrogen.
$ Diazomethane $ when reacting with a $ Phenol $ acts as a base and abstracts a proton from phenol. This results in the formation of $ phenoxide $ ions. After this $ phenoxide $ ion acts as a nucleophile and under this nucleophilic attack $ Anisole $ ether is formed.
The reaction is shown below:

$ Anisole $ is formed in the reaction and nitrogen gas is liberated when $ Diazomethane $ reacts with . $ Phenol $ . This reaction takes place in the presence of $ Fluoroboric $ $ acid $ .
$ Anisole $ or $ methoxybenzene $ is an organic compound which is a colourless liquid. Its smell is reminiscent of anise seed. Due to this smell it is used in making perfumes, flavouring agents as well as solvent.
Note:
We not only use $ Fluoroboric $ $ acid $ to enhance the rate of reaction but also chemicals like $ Sodium{\text{ }}hydroxide $ which increases the rate of reaction. During such reactions, liberation of nitrogen always takes place.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




