
Phenol is acidic because of resonance stabilization of its conjugate base, namely:
A. Phenoxide ion
B. Epoxide ion
C. Benzoate ion
D. None of these
Answer
571.5k+ views
Hint:The acidity of the acid depends upon the stabilization of the conjugate base. The conjugate base forms by the removal of protons. The conjugate base is the ion of the acid.
Complete step by step solution:
A compound that donates protons known as acid. By the donation of a proton a negatively charged species forms which is known as conjugate base. The conjugate base of an acid forms by the removal of a proton or hydrogen.
The structure of the conjugate base of phenol is as follows:
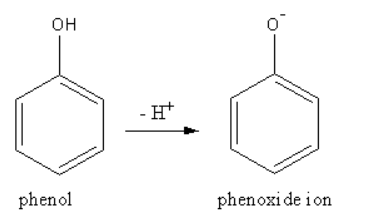
The conjugate base of phenol is named as phenoxide ion.
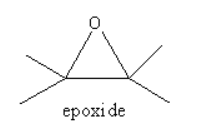
Epoxide ion is formed by epoxide. Epoxides are the ether. The structure of the epoxide is as follows:
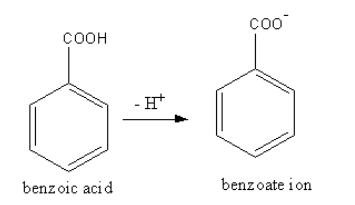
Benzoate ion is the conjugate base of the benzoic acid. Benzoate ion is formed by the removal of a proton from benzoic acid. The structure of the benzoic acid and benzoate ion is as follows:
Phenol is acidic because of resonance stabilization of its, named as phenoxide ion.
Therefore, option (A) phenoxide ion, is correct.
Note:
The name of the ions gives the idea of the acid from which it is formed. The negatively charged oxygen is known as oxide so the phenol is named as phenoxide. When an acid gives a proton it gets a negative charge. The anion is also known as a resonating structure. If the negative charge is stabilized by some factor it increases the stability of the resonating structure hence the acidity of acid. The negative charge of oxygen in phenoxide ion is delocalized in the benzene ring.
Complete step by step solution:
A compound that donates protons known as acid. By the donation of a proton a negatively charged species forms which is known as conjugate base. The conjugate base of an acid forms by the removal of a proton or hydrogen.
The structure of the conjugate base of phenol is as follows:

The conjugate base of phenol is named as phenoxide ion.
Epoxide ion is formed by epoxide. Epoxides are the ether. The structure of the epoxide is as follows:

Benzoate ion is the conjugate base of the benzoic acid. Benzoate ion is formed by the removal of a proton from benzoic acid. The structure of the benzoic acid and benzoate ion is as follows:

Phenol is acidic because of resonance stabilization of its, named as phenoxide ion.
Therefore, option (A) phenoxide ion, is correct.
Note:
The name of the ions gives the idea of the acid from which it is formed. The negatively charged oxygen is known as oxide so the phenol is named as phenoxide. When an acid gives a proton it gets a negative charge. The anion is also known as a resonating structure. If the negative charge is stabilized by some factor it increases the stability of the resonating structure hence the acidity of acid. The negative charge of oxygen in phenoxide ion is delocalized in the benzene ring.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




