
When phenol is treated with bromine water, the product formed is:
A. $o - {\rm{Bromo}}\;{\rm{phenol}}$
B. $p - {\rm{Bromo}}\;{\rm{phenol}}$
C. $m - {\rm{Bromo}}\;{\rm{phenol}}$
D. $2,4,6 - {\rm{Tribromo}}\;{\rm{phenol}}$
Answer
585.3k+ views
Hint: We know that the reaction of phenol with halogen is commonly termed as halogenation reaction. The reaction of phenol with bromine water results in the formation of polyhalogen derivatives.
Complete step by step answer:
It is known to us that if we add bromine water to a solution of phenol. The discoloration of bromine water occurs and the formation of white precipitate takes place. The white precipitate is $2,4,6 - {\rm{Tribromo}}\;{\rm{phenol}}$. In this reaction, the polarity of phenol plays a very important role in the formation of electrophile. In the given case, ${\rm{B}}{{\rm{r}}^ + }$ acts as the electrophile. Basically, it is an electrophilic substitution reaction in which the replacement of hydrogen atom present on the ring of phenol takes place by bromine.
As the substitution is initiated by using an electrophile, the reaction is named as electrophilic substitution. Depending on the electrophile used, ortho, meta or para substitution takes place. The para substitution is favored the most as there will not be any steric hindrance. Electrophilic substitutions in lower temperature prefer para substituted product over ortho product.
The reaction of phenol with bromine water is shown below.
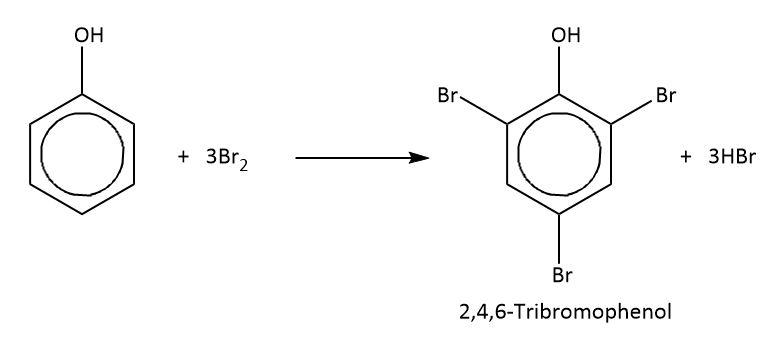
Hence, we can say that, when phenol is treated with bromine water, the product formed is $2,4,6 - {\rm{Tribromo}}\;{\rm{phenol}}$
Thus, the correct option is D.
Note:
As we know that, aromatic compounds undergo electrophilic substitution reactions where an electrophile with a positive charge attacks the ring. The substitution can take place in ortho, meta, or para positions in the ring.
Complete step by step answer:
It is known to us that if we add bromine water to a solution of phenol. The discoloration of bromine water occurs and the formation of white precipitate takes place. The white precipitate is $2,4,6 - {\rm{Tribromo}}\;{\rm{phenol}}$. In this reaction, the polarity of phenol plays a very important role in the formation of electrophile. In the given case, ${\rm{B}}{{\rm{r}}^ + }$ acts as the electrophile. Basically, it is an electrophilic substitution reaction in which the replacement of hydrogen atom present on the ring of phenol takes place by bromine.
As the substitution is initiated by using an electrophile, the reaction is named as electrophilic substitution. Depending on the electrophile used, ortho, meta or para substitution takes place. The para substitution is favored the most as there will not be any steric hindrance. Electrophilic substitutions in lower temperature prefer para substituted product over ortho product.
The reaction of phenol with bromine water is shown below.

Hence, we can say that, when phenol is treated with bromine water, the product formed is $2,4,6 - {\rm{Tribromo}}\;{\rm{phenol}}$
Thus, the correct option is D.
Note:
As we know that, aromatic compounds undergo electrophilic substitution reactions where an electrophile with a positive charge attacks the ring. The substitution can take place in ortho, meta, or para positions in the ring.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




