
Phenol on treating with concentrated ${H_2}S{O_4}$ at $15 - {20^ \circ }C$ mainly produces:
A: phenol$ - 2,4,6 - $trisulfonic acid
B: $2 - $hydroxy benzene sulphonic acid
C: phenol$ - 4 - $ sulphonic acid
D: a $50\% $ mixture of ortho and para phenolsulphonic acid
Answer
585.6k+ views
Hint: In organic reactions, the temperature at which reaction is taking place and the concentration of reactants is very important. By changing the temperature or the concentration of reactants, the product is changed completely.
Complete step by step answer:
Chemical formula of phenol is ${C_6}{H_5}OH$. Phenol reacts with different concentrations of sulphuric acid at different temperatures to yield different products. When phenol is heated at ${100^ \circ }C$ with concentrated sulphuric acid, $4 - $hydroxy benzene sulphonic acid is formed. Reaction taking place is:
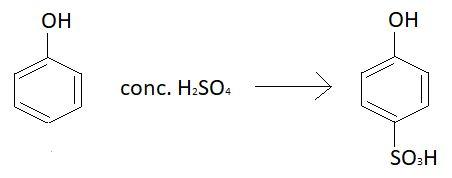
$S{O_3}H$ group is sulphonic acid group and $OH$ group is hydroxyl group.
When phenol will react with concentrated sulphuric acid at $15 - {20^ \circ }C$ product will not be the same as above. At this temperature $2 - $hydroxy benzene sulphonic acid will be formed. Reaction involved at this temperature is:
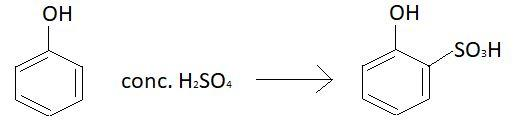
In this case sulphonic acid group will be attached with carbon atom at ortho position and hence the product formed will be $2 - $hydroxy benzene sulphonic acid.
So, the correct answer is Option C.
Additional Information:
Phenol reacts with dilute and concentrated nitric acid. Chemical formula of nitric acid is $HN{O_3}$. In both the reactions products obtained are different. Phenol reacts with dilute nitric acid to give a mixture of ortho and para nitrophenol whereas phenol reacts with concentrated nitric acid to give picric acid or $2,4,6 - $trinitrophenol.
Note:
In benzene ring carbon atom which is present at adjacent position to carbon atom containing functional group is called ortho carbon, carbon which is opposite to carbon atom containing functional group is called para carbon and the carbon between ortho and para is called meta carbon.
Complete step by step answer:
Chemical formula of phenol is ${C_6}{H_5}OH$. Phenol reacts with different concentrations of sulphuric acid at different temperatures to yield different products. When phenol is heated at ${100^ \circ }C$ with concentrated sulphuric acid, $4 - $hydroxy benzene sulphonic acid is formed. Reaction taking place is:
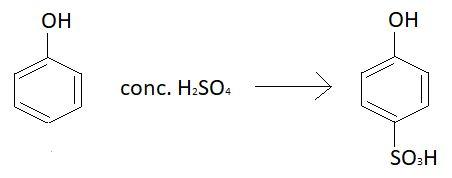
$S{O_3}H$ group is sulphonic acid group and $OH$ group is hydroxyl group.
When phenol will react with concentrated sulphuric acid at $15 - {20^ \circ }C$ product will not be the same as above. At this temperature $2 - $hydroxy benzene sulphonic acid will be formed. Reaction involved at this temperature is:
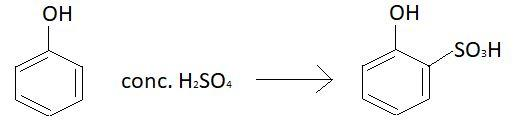
In this case sulphonic acid group will be attached with carbon atom at ortho position and hence the product formed will be $2 - $hydroxy benzene sulphonic acid.
So, the correct answer is Option C.
Additional Information:
Phenol reacts with dilute and concentrated nitric acid. Chemical formula of nitric acid is $HN{O_3}$. In both the reactions products obtained are different. Phenol reacts with dilute nitric acid to give a mixture of ortho and para nitrophenol whereas phenol reacts with concentrated nitric acid to give picric acid or $2,4,6 - $trinitrophenol.
Note:
In benzene ring carbon atom which is present at adjacent position to carbon atom containing functional group is called ortho carbon, carbon which is opposite to carbon atom containing functional group is called para carbon and the carbon between ortho and para is called meta carbon.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




