
How is phenol prepared from
(A).Chlorobenzene
(B).Isopropyl benzene
(C).Benzene sulphonic acid
(D).Aniline
Answer
578.4k+ views
Hint: Phenol is a member of a class of organic compounds having the same name i.e. phenol. Phenol is also referred to as benzenol or carbolic acid.
Complete answer:
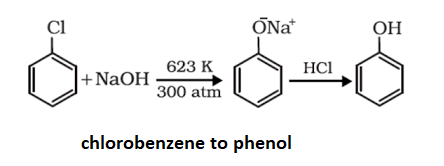
Preparation of phenol from Chlorobenzene:
Phenol is prepared when Chlorobenzene is fused with sodium hydroxide at the temperature range of 623K and pressure around 320 atm. After the fusion of Chlorobenzene with sodium hydroxide sodium phenoxide is produced which on acidification gives phenol.
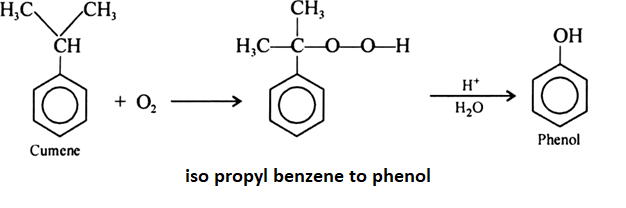
Phenol is prepared from isopropyl benzene by oxidation of isopropyl benzene. Cumene hydroperoxide is formed after the oxidation of isopropyl benzene and then it is hydrolyzed which leads to formation of phenol.
Phenol is prepared from aniline. At the first step aniline is treated with HCL at around 273 to 278 K diazonium salts are obtained. These diazonium salts are very reactive. And when they are warm with water they get hydrolyzed to phenol. Phenols can also be obtained when the diazonium salt is treated with dilute acids.

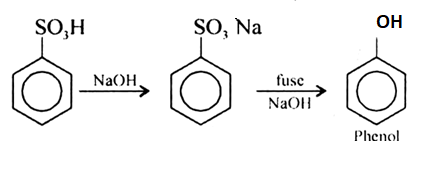
Phenol is prepared from benzene sulphonic acid when the benzene sulphonic acid is treated with the requisite quantity of soda as sodium carbonate or sodium hydroxide around the temperature of 573 K and then sodium phenoxide is formed. Then the sodium phenoxide is treated with Sulphuric acid and produces phenol.
Note:
Phenol is most commonly used as a disinfectant since the ancient time. Phenol also has antiseptic properties too. Phenol in very less quantity is also used in the manufacturing of mouthwashes for effective cleaning of the mouth and the removal of bacteria from the mouth.
Complete answer:
Preparation of phenol from Chlorobenzene:
Phenol is prepared when Chlorobenzene is fused with sodium hydroxide at the temperature range of 623K and pressure around 320 atm. After the fusion of Chlorobenzene with sodium hydroxide sodium phenoxide is produced which on acidification gives phenol.

Phenol is prepared from isopropyl benzene by oxidation of isopropyl benzene. Cumene hydroperoxide is formed after the oxidation of isopropyl benzene and then it is hydrolyzed which leads to formation of phenol.

Phenol is prepared from aniline. At the first step aniline is treated with HCL at around 273 to 278 K diazonium salts are obtained. These diazonium salts are very reactive. And when they are warm with water they get hydrolyzed to phenol. Phenols can also be obtained when the diazonium salt is treated with dilute acids.

Phenol is prepared from benzene sulphonic acid when the benzene sulphonic acid is treated with the requisite quantity of soda as sodium carbonate or sodium hydroxide around the temperature of 573 K and then sodium phenoxide is formed. Then the sodium phenoxide is treated with Sulphuric acid and produces phenol.

Note:
Phenol is most commonly used as a disinfectant since the ancient time. Phenol also has antiseptic properties too. Phenol in very less quantity is also used in the manufacturing of mouthwashes for effective cleaning of the mouth and the removal of bacteria from the mouth.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




