
Phenol, when treated with the excess of bromine water, gives a white precipitate of?
A) 2,4,6-tribromophenol
B) o-bromophenol
C) p-bromophenol
D) bromobenzene
Answer
594.6k+ views
Hint: We know that the reaction of phenol with bromine water is known as bromination of phenol. In this reaction hydrogen atoms of benzene rings of phenol are replaced by bromine (halogen).
Complete answer:
Phenol treated with bromine water produces 2, 4, 6-tribromophenol. The reaction is as follows:
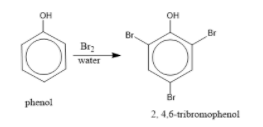
In the bromination reaction of phenol, water is ionized. And also bromine gets ionized to produce bromonium ions to a larger extent. Phenol gets ionized to produce an ortho-para directing phenoxide ion. Bromine water is mostly used as a test for C=C double bond. During this reaction white precipitates form at the end and bromine water is decolourised.
Hence, the formation of bromonium ion and strong ortho-para directing species indicates that the product formation is 2, 4, 6-tribromophenol.
Hence, the correct answer is (A) 2, 4, 6-tribromophenol.
Note: Students might be confused about the ortho-para directing and meta directing groups. Ortho-para directors are activating groups while meta directors are deactivating groups. All electron releasing groups are activating groups and deactivating groups are electron withdrawing.
Complete answer:
Phenol treated with bromine water produces 2, 4, 6-tribromophenol. The reaction is as follows:

In the bromination reaction of phenol, water is ionized. And also bromine gets ionized to produce bromonium ions to a larger extent. Phenol gets ionized to produce an ortho-para directing phenoxide ion. Bromine water is mostly used as a test for C=C double bond. During this reaction white precipitates form at the end and bromine water is decolourised.
Hence, the formation of bromonium ion and strong ortho-para directing species indicates that the product formation is 2, 4, 6-tribromophenol.
Hence, the correct answer is (A) 2, 4, 6-tribromophenol.
Note: Students might be confused about the ortho-para directing and meta directing groups. Ortho-para directors are activating groups while meta directors are deactivating groups. All electron releasing groups are activating groups and deactivating groups are electron withdrawing.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




