
Phenolphthalein gives a pink colour in the alkaline medium due to the fact that:
(A) It is a coloured compound
(B) It ionizes to give coloured ions
(C) It is decomposed by alkali
(D) It forms a complex compound with an alkali
Answer
588.6k+ views
Hint: An attempt to this question can be done by drawing the structure of phenolphthalein and reacting it with an alkali to see how they react and then conclude the reason for colouration of the solution.
Complete step by step solution:
We will first try to understand what exactly is phenolphthalein and its use.
-Phenolphthalein is widely used as an indicator in acid-base titrations.
-It turns colourless in presence of an acid and turns pink in presence of a base.
Now we will draw the structure of phenolphthalein and write its reaction with an alkali to find out the reason for colour change.
Phenolphthalein:
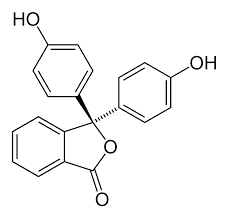
To the solution of phenolphthalein, we will now add a base.
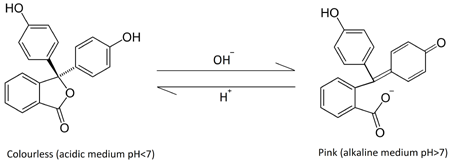
In the above reaction we see that when we add a base, the anion part of the base breaks the C-O bond leading to the formation of ions. It is due to the formation of ions that the solution turns pink.
Therefore, the correct answer is option (B).
Note: Do not get confused with the options given. The above reaction is not a decomposition reaction. The reason for colouration is due to the formation of ions through a chemical reaction. Phenolphthalein is a colourless compound and changes colour only when an alkali is added to it.
Complete step by step solution:
We will first try to understand what exactly is phenolphthalein and its use.
-Phenolphthalein is widely used as an indicator in acid-base titrations.
-It turns colourless in presence of an acid and turns pink in presence of a base.
Now we will draw the structure of phenolphthalein and write its reaction with an alkali to find out the reason for colour change.
Phenolphthalein:

To the solution of phenolphthalein, we will now add a base.

In the above reaction we see that when we add a base, the anion part of the base breaks the C-O bond leading to the formation of ions. It is due to the formation of ions that the solution turns pink.
Therefore, the correct answer is option (B).
Note: Do not get confused with the options given. The above reaction is not a decomposition reaction. The reason for colouration is due to the formation of ions through a chemical reaction. Phenolphthalein is a colourless compound and changes colour only when an alkali is added to it.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




