
When phenolphthalein gives pink colour in the given salt solution the salt solution will be:
(A) Acidic
(B) Basic
(C) Neutral
(D) Phenolphthalein does not given pink colour in solution
Answer
588k+ views
Hint: Phenolphthalein is an indicator of acid base titrations. Its chemical formula is ${C_{20}}{H_{14}}{O_4}$ and it is commonly abbreviated as it gives different solute in aside and basic medium and helps in indicating the completion of acid base titration.
Complete step by step answer:
During titrations phenolphthalein undergoes four changes in its form.
(1) In strong acidic conditions it is protonated to form and gives orange change color.
(2) In intermediately acidic HIN which is colorless.
(3) In basic condition it gets doubly protonated of form $1{n^{2 - }}$ and gives pink color.
(4)In strong basic conditions it forms $in{\left( {OH} \right)^{3 - }}$ and its pink color fades solely generally at a $PH$ about 13.
The pink color of phenolphthalein is observed when its $pH$ is $8.5 - 9.0$ thus a basic $PH$.
Phenolphthalein is soluble in alcohols according to le hotelier phenolphthalein or vice versa, it leads to more ionization and its equilibrium shifts.
Other indicators that are commonly used are thinly blue, methyl red, bromate mole blue etc.
Hence the correct option is B.
Additional information: Phenolphthalein is used as a disappearing ink in toys. Phenolphthalein is mixed with sodium hydroxide to make the ink. When this ink reacts with carbon dioxide in air, it’s pH gets reduced and becomes colourless. It is also used as an artificial blood stain in movies and stage shows.
Note:
In 1871 Adolf Baeyer synthesized phenolphthalein by condensation of two equivalent pends with phallic anhydride under acidic environment
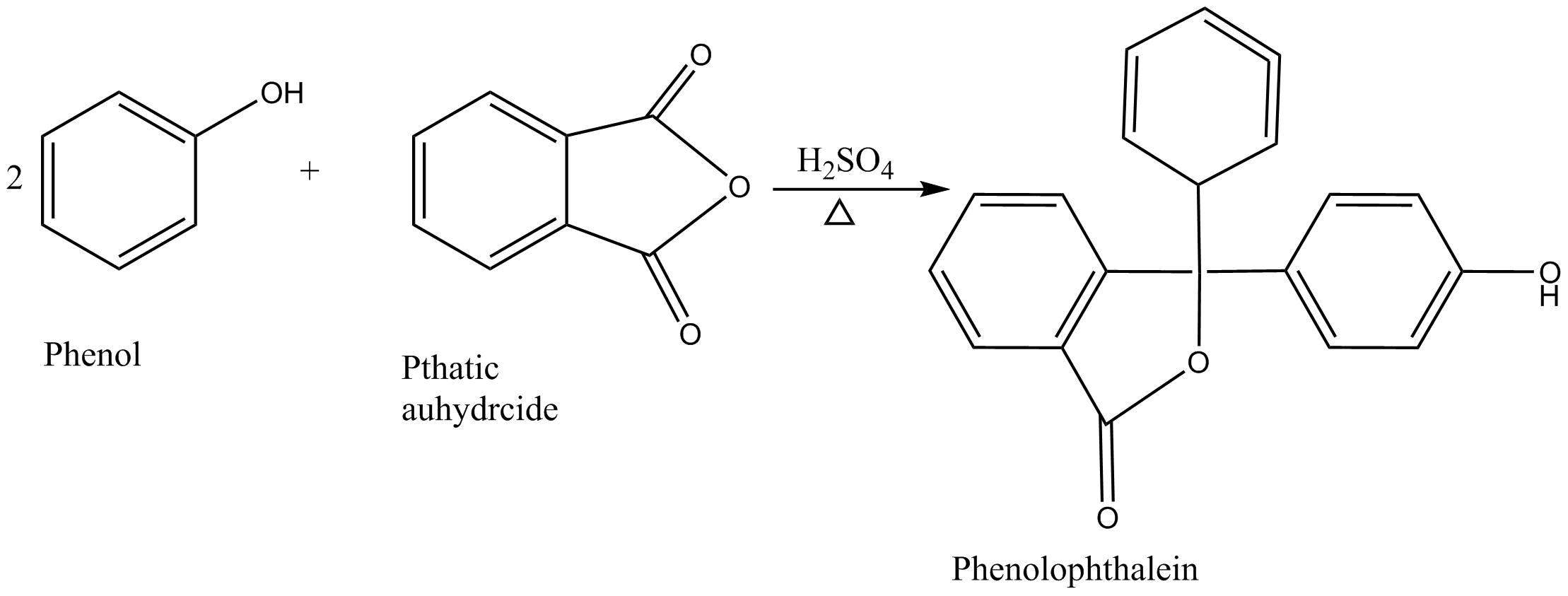
Phenolphthalein derived its name from this reaction.
Complete step by step answer:
During titrations phenolphthalein undergoes four changes in its form.
(1) In strong acidic conditions it is protonated to form and gives orange change color.
(2) In intermediately acidic HIN which is colorless.
(3) In basic condition it gets doubly protonated of form $1{n^{2 - }}$ and gives pink color.
(4)In strong basic conditions it forms $in{\left( {OH} \right)^{3 - }}$ and its pink color fades solely generally at a $PH$ about 13.
The pink color of phenolphthalein is observed when its $pH$ is $8.5 - 9.0$ thus a basic $PH$.
Phenolphthalein is soluble in alcohols according to le hotelier phenolphthalein or vice versa, it leads to more ionization and its equilibrium shifts.
Other indicators that are commonly used are thinly blue, methyl red, bromate mole blue etc.
Hence the correct option is B.
Additional information: Phenolphthalein is used as a disappearing ink in toys. Phenolphthalein is mixed with sodium hydroxide to make the ink. When this ink reacts with carbon dioxide in air, it’s pH gets reduced and becomes colourless. It is also used as an artificial blood stain in movies and stage shows.
Note:
In 1871 Adolf Baeyer synthesized phenolphthalein by condensation of two equivalent pends with phallic anhydride under acidic environment

Phenolphthalein derived its name from this reaction.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




