
Phenoxide ion has _________ number of resonating structures than benzoate ion and benzoic acid is a __________ acid than phenol.
A. More, stronger
B. More, weaker
C. Less, stronger
D. Less, weaker
Answer
595.8k+ views
Hint: Two or more Lewis structures that represent collectively a single species are known as resonating structures. First, we have to draw the reasoning structures of both benzoate ion and benzoic acid.
Complete step-by-step answer:
We first draw the structure of phenoxide ions.
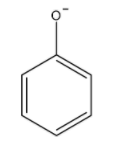
Now, we draw the resonating structure of phenoxide ions. The -ive charge on oxygen atom delocalized over carbon atoms of the benzene ring.
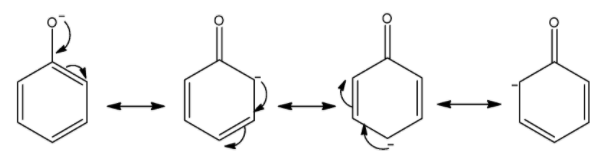
So, there are three resonating structures of phenoxide ions.
Now, for benzoate ions we draw resonating structures. The -ive charge on oxygen atom delocalized over one oxygen atom.
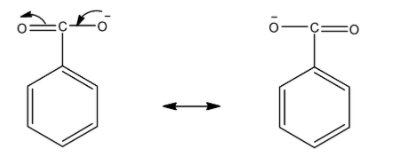
So, there is only one resonating structure of benzoate ions.
Hence, more resonating structures can be drawn for phenoxide ion than benzoate ion.
In phenoxide ion, the negative charge is delocalized over the carbon atoms of the benzene ring but in benzoate ion, the negative charge is delocalized over one oxygen atom. As the oxygen atom is more electronegative than carbon atom, it can donate protons easily. So, the acidity of phenol is less than benzoic acid.
Hence, option A is correct.
Note: Students might think that as phenoxide ion has more number of resonating structures than benzoate ion, phenol is more acidic than benzoic acid. But it is incorrect. The negative charge on less electronegative carbon atoms makes phenol less acidic than benzoic acid.
Complete step-by-step answer:
We first draw the structure of phenoxide ions.

Now, we draw the resonating structure of phenoxide ions. The -ive charge on oxygen atom delocalized over carbon atoms of the benzene ring.

So, there are three resonating structures of phenoxide ions.
Now, for benzoate ions we draw resonating structures. The -ive charge on oxygen atom delocalized over one oxygen atom.

So, there is only one resonating structure of benzoate ions.
Hence, more resonating structures can be drawn for phenoxide ion than benzoate ion.
In phenoxide ion, the negative charge is delocalized over the carbon atoms of the benzene ring but in benzoate ion, the negative charge is delocalized over one oxygen atom. As the oxygen atom is more electronegative than carbon atom, it can donate protons easily. So, the acidity of phenol is less than benzoic acid.
Hence, option A is correct.
Note: Students might think that as phenoxide ion has more number of resonating structures than benzoate ion, phenol is more acidic than benzoic acid. But it is incorrect. The negative charge on less electronegative carbon atoms makes phenol less acidic than benzoic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




