
Why is phenyl carbocation unstable?
Answer
492.6k+ views
Hint: To solve this question we should know about:
A chemical that is stable is unreactive, while an unstable chemical is reactive. The Unequal Distribution of Electrons (UDED) in a chemical species determines reactivity (molecule, atom, and ion). This is how "unreactive" chemical species behave. Like the compressed atmosphere in a sealed cylinder, their electrons are securely confined by their nuclei. To make it explode, you'll probably need a big fire (a lot of energy) in the room (react)
Complete answer:
The creation of a phenyl cation might be thought of as
${C_6}{H_5} - H \to {C_6}{H_5} + H + {e^ - }$
The benzene $C - H$ bonds are $s{p^2}$ hybridized.
Because the electrons are closer to the nucleus with a high s character, we must use more energy to remove them and break the bond.
Ethane, for example, requires \[423KJmo{l^{ - 1}}\] to break the C-H bond, but benzene requires \[470KJmo{l^{ - 1}}\] .
Furthermore, the unoccupied $s{p^2}$ orbital is in the ring's plane.
Because it can't overlap with the system's $\pi $ orbitals, it can't be stabilized via resonance.
The phenyl cation is an unstable, high-energy species.
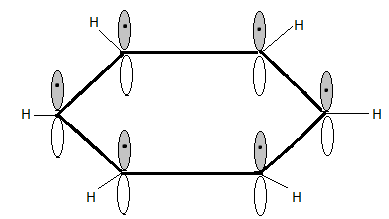
Because of the high bond energy of the aromatic $C - H$ bond, the phenyl carbocation is unstable.
Note:
Because the phenyl cation core has two ligands but no lone pairs, $sp$ is the most stable hybridization. Of course, it's not genuinely $sp$ because it can't take on a linear geometry, but it's also not $s{p^2}$ because the bond angle is greater than 120°.
A chemical that is stable is unreactive, while an unstable chemical is reactive. The Unequal Distribution of Electrons (UDED) in a chemical species determines reactivity (molecule, atom, and ion). This is how "unreactive" chemical species behave. Like the compressed atmosphere in a sealed cylinder, their electrons are securely confined by their nuclei. To make it explode, you'll probably need a big fire (a lot of energy) in the room (react)
Complete answer:
The creation of a phenyl cation might be thought of as
${C_6}{H_5} - H \to {C_6}{H_5} + H + {e^ - }$
The benzene $C - H$ bonds are $s{p^2}$ hybridized.
Because the electrons are closer to the nucleus with a high s character, we must use more energy to remove them and break the bond.
Ethane, for example, requires \[423KJmo{l^{ - 1}}\] to break the C-H bond, but benzene requires \[470KJmo{l^{ - 1}}\] .
Furthermore, the unoccupied $s{p^2}$ orbital is in the ring's plane.
Because it can't overlap with the system's $\pi $ orbitals, it can't be stabilized via resonance.
The phenyl cation is an unstable, high-energy species.

Because of the high bond energy of the aromatic $C - H$ bond, the phenyl carbocation is unstable.
Note:
Because the phenyl cation core has two ligands but no lone pairs, $sp$ is the most stable hybridization. Of course, it's not genuinely $sp$ because it can't take on a linear geometry, but it's also not $s{p^2}$ because the bond angle is greater than 120°.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




