
Phenyl magnesium bromide reacts with methanol to give
A) A mixture of anisole and \[{\text{Mg(OH)Br}}\]
B) A mixture of benzene and \[{\text{Mg(OMe)Br}}\]
C) A mixture of toluene and \[{\text{Mg(OH)Br}}\]
D) A mixture of phenol and\[{\text{Mg(Me)Br}}\]
Answer
564.9k+ views
Hint: This is the reaction of the Grignard reagent with alcohol. It is known as the Zerewitnoff reaction. The reaction of phenyl magnesium bromide with methanol is an acid-base reaction. Methanol behaves as acid while phenyl magnesium bromide behaves as a base.
Complete step by step answer:
Phenyl magnesium bromide is a Grignard reagent.
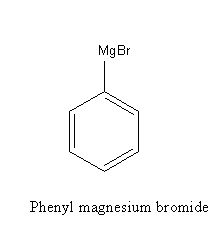
The Grignard reagent is an organometallic compound.
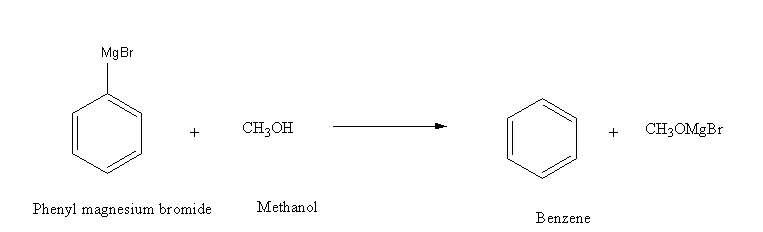 This is an acid-base reaction when methanol acts as an acid and donates its proton. While phenyl magnesium bromide acts as a base and accepts the proton and is converted into benzene.
This is an acid-base reaction when methanol acts as an acid and donates its proton. While phenyl magnesium bromide acts as a base and accepts the proton and is converted into benzene.
Thus, Phenyl magnesium bromide reacts with methanol and gives a mixture of benzene and\[{\text{Mg(OMe)Br}}\].
Therefore, option (B) is correct.
Additional Information:
1) The Grignard reagent is a good nucleophile. It acts as a nucleophile in reaction with a carbonyl compound. It can form a new carbon-carbon bond. It behaves as a base in reaction with alcohol. The reaction of an alcohol with Grignard reagent is different than the reaction with a carbonyl compound.
2) In phenyl magnesium bromide, phenyl has a partial negative charge and magnesium has a partial positive charge. In methanol, the methoxide group has a partial negative charge while hydrogen has a partial positive charge. So phenyl attacks the acidic hydrogen of methanol and gives benzene as the product.
Note:Zerewitnoff reaction used to determine the active hydrogen in an organic compound. Alkyl magnesium bromides react with alcohol and give alkane as the product. While phenyl magnesium bromide reacts with alcohol and gives benzene as the product.
Complete step by step answer:
Phenyl magnesium bromide is a Grignard reagent.

The Grignard reagent is an organometallic compound.

Thus, Phenyl magnesium bromide reacts with methanol and gives a mixture of benzene and\[{\text{Mg(OMe)Br}}\].
Therefore, option (B) is correct.
Additional Information:
1) The Grignard reagent is a good nucleophile. It acts as a nucleophile in reaction with a carbonyl compound. It can form a new carbon-carbon bond. It behaves as a base in reaction with alcohol. The reaction of an alcohol with Grignard reagent is different than the reaction with a carbonyl compound.
2) In phenyl magnesium bromide, phenyl has a partial negative charge and magnesium has a partial positive charge. In methanol, the methoxide group has a partial negative charge while hydrogen has a partial positive charge. So phenyl attacks the acidic hydrogen of methanol and gives benzene as the product.
Note:Zerewitnoff reaction used to determine the active hydrogen in an organic compound. Alkyl magnesium bromides react with alcohol and give alkane as the product. While phenyl magnesium bromide reacts with alcohol and gives benzene as the product.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




