
Phenylacetate can be prepared by the reaction of
A. Acetic acid and phenol
B. Acetyl chloride and phenol
C. Acetyl chloride and benzene
D. Sodium phenate and methyl iodide
Answer
568.8k+ views
Hint: To solve such problems,we should have proper knowledge of basic molecules and their chemical formula. Acylation of phenol gives phenylacetate. Phenols are less reactive than alcohol so reactions used for esterification of alcohol cannot be used for phenol.
Complete step by step answer:As we know that alcohols react with carboxylic acids in presence of an acid catalyst and give ester as the product.
a) Acetic acid and phenol
In the case of the phenol hydroxyl group is directly bonded to a benzene ring so the lone pair electrons on oxygen atom are distributed to the ring. So, phenol is less reactive than alcohol.
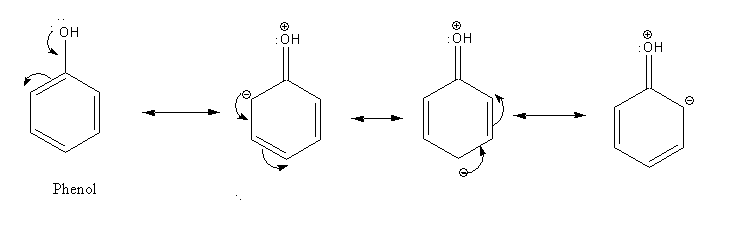
Due to the delocalization of electrons, phenolic oxygen is a weak nucleophile to attack on acetic acid. Thus, due to less reactivity of phenol, it does not react with acetic acid to give phenylacetate as the product.
Hence, Option (A) is an incorrect answer.
b) Acetyl chloride and phenol
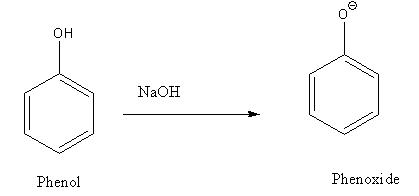
Phenol reacts with acetyl chloride in an aqueous \[{\text{NaOH}}\] solution to form phenylacetate. The \[{\text{NaOH}}\] converts phenol to more nucleophilic phenoxide ions.
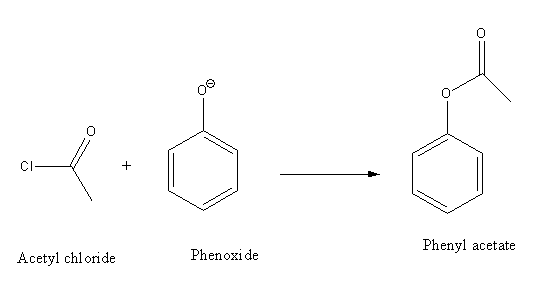
Thus, option (B) Acetyl chloride and phenol is the correct answer.
c) Acetyl chloride and benzene
Acetyl chloride reacts with benzene in presence of \[{\text{AlC}}{{\text{l}}_{\text{3}}}\] a catalyst and gives acetophenone as the product.
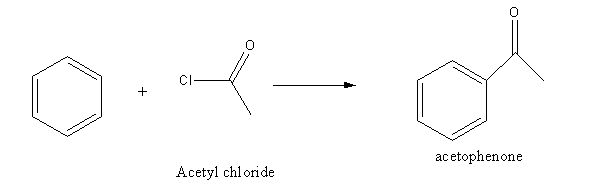
Hence, Option (C) is an incorrect answer.
d) Sodium phenate and methyl iodide
Sodium phenate reacts with methyl iodide and gives phenyl methyl ether as the product.
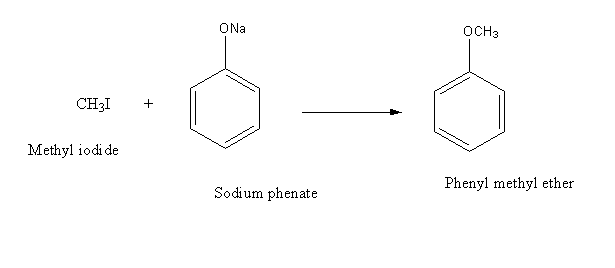
Hence, Option (D) is an incorrect answer.
The correct answer is (B)
Note: Though both phenol and alcohol contain hydroxyl groups but the hydroxyl group of phenol is less nucleophilic than the hydroxyl group of alcohol. So phenol shows different reactions than alcohol. Alcohol gives ester on reaction with carboxylic acid however, phenol does not give ester on reaction with a carboxylic acid.
Complete step by step answer:As we know that alcohols react with carboxylic acids in presence of an acid catalyst and give ester as the product.
a) Acetic acid and phenol
In the case of the phenol hydroxyl group is directly bonded to a benzene ring so the lone pair electrons on oxygen atom are distributed to the ring. So, phenol is less reactive than alcohol.

Due to the delocalization of electrons, phenolic oxygen is a weak nucleophile to attack on acetic acid. Thus, due to less reactivity of phenol, it does not react with acetic acid to give phenylacetate as the product.
Hence, Option (A) is an incorrect answer.
b) Acetyl chloride and phenol
Phenol reacts with acetyl chloride in an aqueous \[{\text{NaOH}}\] solution to form phenylacetate. The \[{\text{NaOH}}\] converts phenol to more nucleophilic phenoxide ions.


Thus, option (B) Acetyl chloride and phenol is the correct answer.
c) Acetyl chloride and benzene
Acetyl chloride reacts with benzene in presence of \[{\text{AlC}}{{\text{l}}_{\text{3}}}\] a catalyst and gives acetophenone as the product.

Hence, Option (C) is an incorrect answer.
d) Sodium phenate and methyl iodide
Sodium phenate reacts with methyl iodide and gives phenyl methyl ether as the product.

Hence, Option (D) is an incorrect answer.
The correct answer is (B)
Note: Though both phenol and alcohol contain hydroxyl groups but the hydroxyl group of phenol is less nucleophilic than the hydroxyl group of alcohol. So phenol shows different reactions than alcohol. Alcohol gives ester on reaction with carboxylic acid however, phenol does not give ester on reaction with a carboxylic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




