
What is photoelectric effect? Explain the effect of increase (i) frequency (ii) intensity of incident radiation on photoelectric current with suitable graphs.
Answer
595.5k+ views
Hint: The emission of electrons from a metal surface by means of electromagnetic radiations of suitable high frequency is called photoelectric effect. A suitable frequency is required for the production of photocurrent and the intensity of light.
Complete step-by-step answer:
(i). The phenomena of emission of electrons from a metallic surface when it is subjected to electromagnetic radiation of sufficiently high frequency is incident on it, is called the photoelectric effect. The photo (light) generated electrons are called photoelectrons.
This process is known as photoelectric emission and it is an instantaneous process. The time lag between the incidence of light radiation and the emission of a photon is very small, even less than${10^{-9}}$s . The value of retarding potential at which the photoelectric current becomes zero is called cut off potential or stopping potential for the given frequency of the incident radiation.
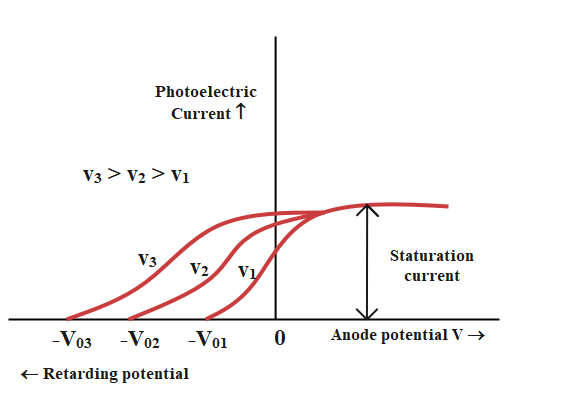
To study the effect of frequency on the photoelectric effect, the intensity of incident radiation is adjusted in such a way that the saturation current is the same each time. It is observed that for increasing frequency the stopping potentials also tend to increase. This implies that greater the frequency of the incident radiation, greater is the maximum kinetic energy of the photoelectrons and hence greater is the retarding potential required to stop such electrons completely. Thus frequency only limits the kinetic energy of the emitted photon. But still, a suitable frequency of radiation is required to initiate the process of photon emission, known as the threshold frequency.
The following graph shows the variation of photoelectric with anode potential for different frequencies of incident radiation:
(ii). If we allow radiations of a fixed frequency to fall on any photosensitive material and the accelerating potential between the two electrodes is kept fixed then the photoelectric current is found to be increasing linearly with the intensity of radiation. Since the photoelectric current is directly proportional to the number of photoelectrons emitted per second, this implies that the number of photoelectrons emitted per second is proportional to the intensity of the incident radiation.
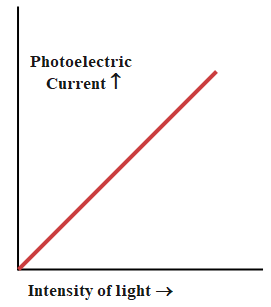
The following graph shows photoelectric current vs intensity of incident radiation.
Note: Above the threshold frequency of a photosensitive material, the stopping potential or equivalently the maximum kinetic energy of the photoelectrons is directly proportional to the frequency of the incident radiation, but is independent of its intensity.
Complete step-by-step answer:
(i). The phenomena of emission of electrons from a metallic surface when it is subjected to electromagnetic radiation of sufficiently high frequency is incident on it, is called the photoelectric effect. The photo (light) generated electrons are called photoelectrons.
This process is known as photoelectric emission and it is an instantaneous process. The time lag between the incidence of light radiation and the emission of a photon is very small, even less than${10^{-9}}$s . The value of retarding potential at which the photoelectric current becomes zero is called cut off potential or stopping potential for the given frequency of the incident radiation.
To study the effect of frequency on the photoelectric effect, the intensity of incident radiation is adjusted in such a way that the saturation current is the same each time. It is observed that for increasing frequency the stopping potentials also tend to increase. This implies that greater the frequency of the incident radiation, greater is the maximum kinetic energy of the photoelectrons and hence greater is the retarding potential required to stop such electrons completely. Thus frequency only limits the kinetic energy of the emitted photon. But still, a suitable frequency of radiation is required to initiate the process of photon emission, known as the threshold frequency.
The following graph shows the variation of photoelectric with anode potential for different frequencies of incident radiation:

(ii). If we allow radiations of a fixed frequency to fall on any photosensitive material and the accelerating potential between the two electrodes is kept fixed then the photoelectric current is found to be increasing linearly with the intensity of radiation. Since the photoelectric current is directly proportional to the number of photoelectrons emitted per second, this implies that the number of photoelectrons emitted per second is proportional to the intensity of the incident radiation.

The following graph shows photoelectric current vs intensity of incident radiation.
Note: Above the threshold frequency of a photosensitive material, the stopping potential or equivalently the maximum kinetic energy of the photoelectrons is directly proportional to the frequency of the incident radiation, but is independent of its intensity.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




