
What is the physical state of water at ${0^ \circ }C$ ?
Answer
591.6k+ views
Hint: Physical state of a substance or chemical species represents whether the given substance or chemical species is in solid, liquid or gaseous state.
Complete step by step answer:
We normally find water in liquid state, but it can easily change its state to solid as well as gas. The interconversion of states can be understood clearly by the following diagram-
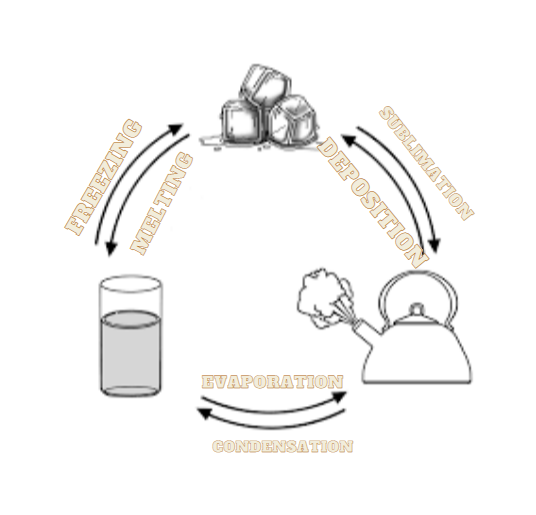
The heat of fusion of a substance, also termed as enthalpy of fusion is the change in the heat content, resulting from providing energy. The heat provided is responsible for changing the state of the substance from solid to liquid at a constant pressure.
Water can exist as solid as well as liquid at ${0^ \circ }C$. If heat equals latent heat of fusion is applied to the solid form of water at this temperature, then the solid state (ice) will be converted to liquid state, i.e., water.
Note:
Water is an inorganic, colorless chemical substance, having chemical formula $H2O$. It has the most remarkable property of dissolving other substances in it and by virtue of this property, it is termed as a universal solvent. Water is amphoteric in nature, i.e., it has the ability to react both with acids as well as bases. The following reactions shows its amphoteric nature-
Acidic behavior- $H2O(l) + NH3 (aq.) \rightleftharpoons H3{O^ + }(aq.) + NH{4^ + }(aq.)$
Basic behavior- $H2O(l) + H2S (aq.) \rightleftharpoons H3{O^ + }(aq.) + H{S^ - }(aq.)$
Boiling point of water is ${100^ \circ }C$ while melting point is ${0^ \circ }C$
Complete step by step answer:
We normally find water in liquid state, but it can easily change its state to solid as well as gas. The interconversion of states can be understood clearly by the following diagram-

The heat of fusion of a substance, also termed as enthalpy of fusion is the change in the heat content, resulting from providing energy. The heat provided is responsible for changing the state of the substance from solid to liquid at a constant pressure.
Water can exist as solid as well as liquid at ${0^ \circ }C$. If heat equals latent heat of fusion is applied to the solid form of water at this temperature, then the solid state (ice) will be converted to liquid state, i.e., water.
Note:
Water is an inorganic, colorless chemical substance, having chemical formula $H2O$. It has the most remarkable property of dissolving other substances in it and by virtue of this property, it is termed as a universal solvent. Water is amphoteric in nature, i.e., it has the ability to react both with acids as well as bases. The following reactions shows its amphoteric nature-
Acidic behavior- $H2O(l) + NH3 (aq.) \rightleftharpoons H3{O^ + }(aq.) + NH{4^ + }(aq.)$
Basic behavior- $H2O(l) + H2S (aq.) \rightleftharpoons H3{O^ + }(aq.) + H{S^ - }(aq.)$
Boiling point of water is ${100^ \circ }C$ while melting point is ${0^ \circ }C$
Recently Updated Pages
Master Class 9 General Knowledge: Engaging Questions & Answers for Success

Master Class 9 Social Science: Engaging Questions & Answers for Success

Master Class 9 English: Engaging Questions & Answers for Success

Master Class 9 Maths: Engaging Questions & Answers for Success

Master Class 9 Science: Engaging Questions & Answers for Success

Class 9 Question and Answer - Your Ultimate Solutions Guide

Trending doubts
Difference Between Plant Cell and Animal Cell

Fill the blanks with the suitable prepositions 1 The class 9 english CBSE

Who is eligible for RTE class 9 social science CBSE

Which places in India experience sunrise first and class 9 social science CBSE

What is pollution? How many types of pollution? Define it

Name 10 Living and Non living things class 9 biology CBSE




