
Piperidine will give azo dye test.
Answer
587.7k+ views
Hint: Write down the expanded structure of the organic compound piperidine. Now identify the functional group present. Azo dye test also called coupling reaction is used to identify the presence of primary aromatic amines. So, check if piperidine is aromatic and has a primary amine functional group.
Complete step-by-step answer:
Piperidine is an organic compound with the molecular formula ${{\text{(C}{{\text{H}}_{2}})}_{5}}\text{NH}$. It is a heterocyclic amine consisting of a six membered ring with 5 methylene bridge groups and one amine group. It exists as a colourless liquid and has characteristic odour of amines.
Piperidine is derived from the Latin word Piper, which means pepper.
We will now draw the expanded structure of piperidine.
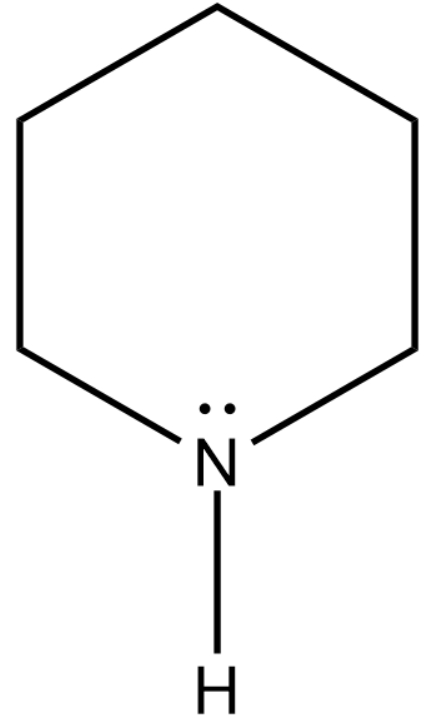
The above compound consists of a secondary amine i.e. two carbon atoms attached to the nitrogen atom.
Azo dye test is answered by primary amine only. This is because the coupling reaction takes place when two hydrogen atoms are attached to a nitrogen atom i.e. primary amine group.
Therefore, piperidine does not give the azo dye test and the correct answer is option (B).
Note: Piperidine and its derivatives are ubiquitous blocks or components of pharmaceutical chemicals. Piperidine structure is commonly found in :
- Icaridin which is a insect repellent
- Selective serotonin reuptake inhibitors
- Stimulants and nootropics like methylphenidate, ethylphenidate, pipradrol etc
- Selective estrogen receptor modulators
- Vasodilators
- Antipsychotic medications
- Opioids
- Arylcyclohexylamines
Along with this piperidine is used in chemical degradation reactions such as in the sequencing of DNA in the opening(cleavage) of modified nucleotides.
Complete step-by-step answer:
Piperidine is an organic compound with the molecular formula ${{\text{(C}{{\text{H}}_{2}})}_{5}}\text{NH}$. It is a heterocyclic amine consisting of a six membered ring with 5 methylene bridge groups and one amine group. It exists as a colourless liquid and has characteristic odour of amines.
Piperidine is derived from the Latin word Piper, which means pepper.
We will now draw the expanded structure of piperidine.

The above compound consists of a secondary amine i.e. two carbon atoms attached to the nitrogen atom.
Azo dye test is answered by primary amine only. This is because the coupling reaction takes place when two hydrogen atoms are attached to a nitrogen atom i.e. primary amine group.
Therefore, piperidine does not give the azo dye test and the correct answer is option (B).
Note: Piperidine and its derivatives are ubiquitous blocks or components of pharmaceutical chemicals. Piperidine structure is commonly found in :
- Icaridin which is a insect repellent
- Selective serotonin reuptake inhibitors
- Stimulants and nootropics like methylphenidate, ethylphenidate, pipradrol etc
- Selective estrogen receptor modulators
- Vasodilators
- Antipsychotic medications
- Opioids
- Arylcyclohexylamines
Along with this piperidine is used in chemical degradation reactions such as in the sequencing of DNA in the opening(cleavage) of modified nucleotides.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




