
Plot the following graphs:
1. $P$ vs $V$
2. $P$ vs $\dfrac{1}{V}$
3. $PV$ vs $P$
Answer
534.6k+ views
Hint: Boyle’s law helps in understanding the behaviour of gases when the temperature is constant. It states that the pressure of the gas is inversely proportional to its volume when the temperature is constant.
$
P\propto V \\
P = \dfrac{k}{V} \\
PV = k \\
$Where $V$ is the volume, $P$ is the pressure and $k$ is the constant value
Complete step by step solution:
1. $P$ vs $V$:
As we know that pressure and volume of a gas is inversely proportional (According to Boyle’s law), Hence, pressure will increase as the volume decreases. The graph is as follows:
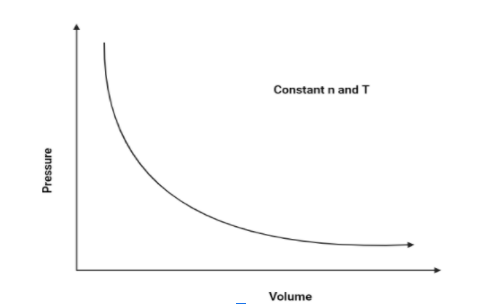
Hence, The graph of pressure vs volume will be a rectangular hyperbola.
2. $P$ vs $\dfrac{1}{V}$:
As we know that $P$ and $\dfrac{1}{V}$ is directly proportional (According to boyle’s law), Hence, pressure will increase as the volume increases.The graph is as follows:
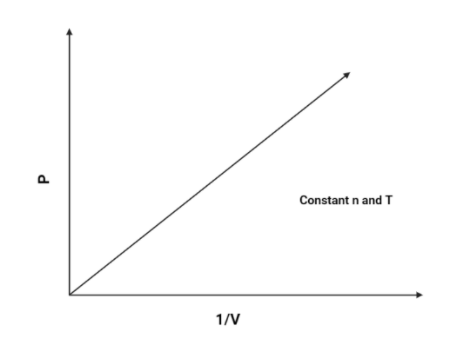
Hence, The graph of $P$ vs $\dfrac{1}{V}$ will be a linear graph.
3. $PV$ vs $P$:
As we know that $PV = k$, (According to Boyle's law). Hence the $PV$ axis will remain constant for all values of $P$. The graph is as follows:
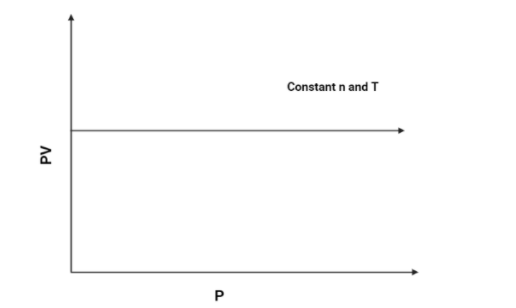
Hence, The graph of $PV$ vs $P$ will be a constant graph.
Note: Boyle’s law has many real life applications. For example, It’s being used in the medical field to check the breathing system in the human body. It helps in explaining how the air pressure within our lungs increases or decreases with respect to the volume of our lungs. This results in the difference in air pressure within our lungs and the environmental air.
$
P\propto V \\
P = \dfrac{k}{V} \\
PV = k \\
$Where $V$ is the volume, $P$ is the pressure and $k$ is the constant value
Complete step by step solution:
1. $P$ vs $V$:
As we know that pressure and volume of a gas is inversely proportional (According to Boyle’s law), Hence, pressure will increase as the volume decreases. The graph is as follows:

Hence, The graph of pressure vs volume will be a rectangular hyperbola.
2. $P$ vs $\dfrac{1}{V}$:
As we know that $P$ and $\dfrac{1}{V}$ is directly proportional (According to boyle’s law), Hence, pressure will increase as the volume increases.The graph is as follows:

Hence, The graph of $P$ vs $\dfrac{1}{V}$ will be a linear graph.
3. $PV$ vs $P$:
As we know that $PV = k$, (According to Boyle's law). Hence the $PV$ axis will remain constant for all values of $P$. The graph is as follows:

Hence, The graph of $PV$ vs $P$ will be a constant graph.
Note: Boyle’s law has many real life applications. For example, It’s being used in the medical field to check the breathing system in the human body. It helps in explaining how the air pressure within our lungs increases or decreases with respect to the volume of our lungs. This results in the difference in air pressure within our lungs and the environmental air.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




