
‘plum pudding’ model of an atom was proposed by
A. C.V.Raman
B. N.Bhor
C. E.Rutherford
D. J.J.Thomson
Answer
595.5k+ views
Hint: First of all we must know that the ‘plum pudding’ model of an atom is one of the first attempts for an atomic model. It is also compared as a watermelon model, raisin pudding model etc. this model was proposed even before the discovery of nucleus.
Complete solution:
Thomson’s Model of Atom (plum pudding model):
As we could see from the heading, it is very clear that it was J.J Thomson who suggested this atomic model. It was in 1898 he suggested that an atom is a positively charged solid sphere and sufficient number of electrons are embedded on it to make the atom electrically neutral.
We could compare this model to a pudding where plums are embedded over it as decoration.it is even compared to a watermelon where the seeds are compared as electrons.
At that time this model was widely accepted because it explains several observations at that time.it could even explain why only negatively charged particles are emitted when a metal is heated and never the positively charged particles.
If we try to build a diagram for this model, it will be as follows. Where negatively charged electrons are embedded a large positively charged body.
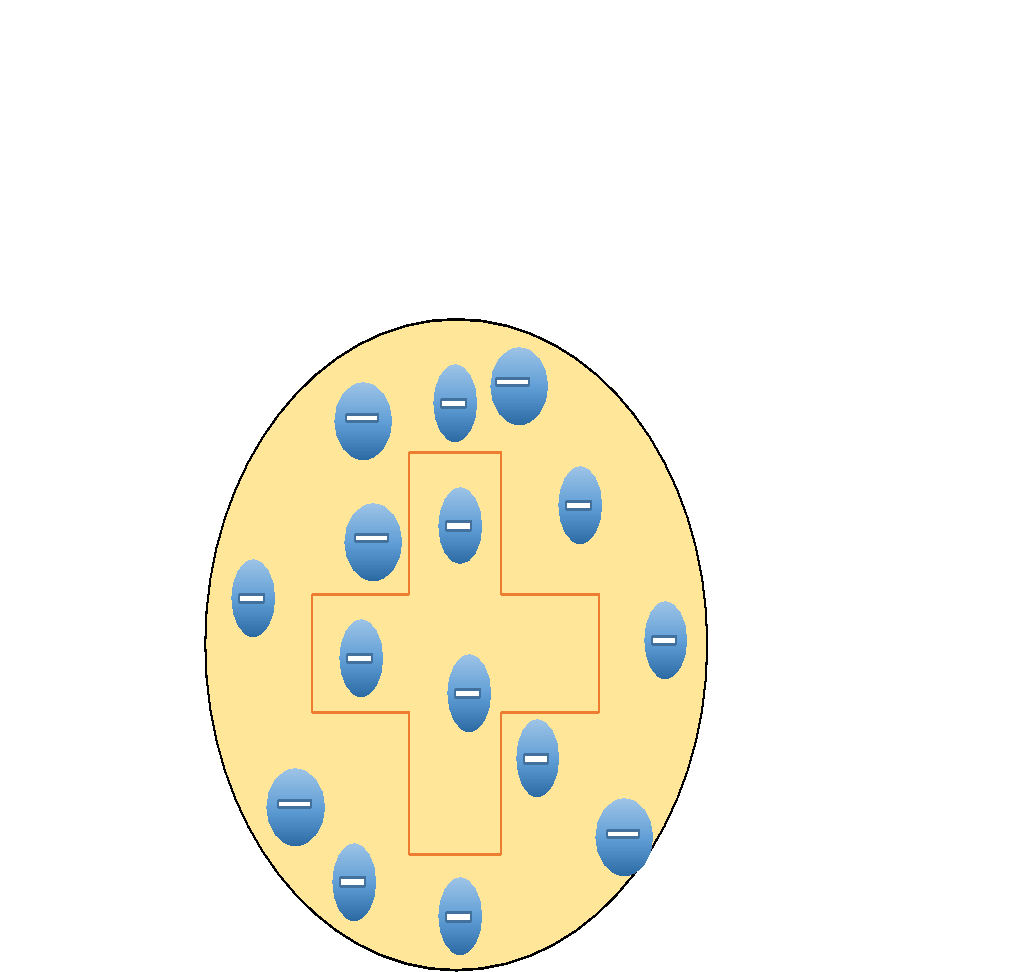
While looking at the Limitations of Thomson model of atom, they are,
1. It failed to explain the atomic stability that how electrons hold on to the positive charge
2. There was nothing mentioned about a nucleus.
So from the options, the correct answer is option d.
Note: There were many atomic models succeeding this plum pudding model. While we look at the chronological order we can find the time of Thompson and other scientists. So this question will be easier if we know the plum pudding model is one of the primary atomic models. Also keep in mind that there is no reference of a nucleus. So we can easily predict this was even before the discovery of nucleus.
Complete solution:
Thomson’s Model of Atom (plum pudding model):
As we could see from the heading, it is very clear that it was J.J Thomson who suggested this atomic model. It was in 1898 he suggested that an atom is a positively charged solid sphere and sufficient number of electrons are embedded on it to make the atom electrically neutral.
We could compare this model to a pudding where plums are embedded over it as decoration.it is even compared to a watermelon where the seeds are compared as electrons.
At that time this model was widely accepted because it explains several observations at that time.it could even explain why only negatively charged particles are emitted when a metal is heated and never the positively charged particles.
If we try to build a diagram for this model, it will be as follows. Where negatively charged electrons are embedded a large positively charged body.

While looking at the Limitations of Thomson model of atom, they are,
1. It failed to explain the atomic stability that how electrons hold on to the positive charge
2. There was nothing mentioned about a nucleus.
So from the options, the correct answer is option d.
Note: There were many atomic models succeeding this plum pudding model. While we look at the chronological order we can find the time of Thompson and other scientists. So this question will be easier if we know the plum pudding model is one of the primary atomic models. Also keep in mind that there is no reference of a nucleus. So we can easily predict this was even before the discovery of nucleus.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

Give 10 examples of unisexual and bisexual flowers




