
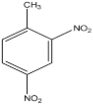
p-Nitrotoluene on further nitration gives:
A.
B.
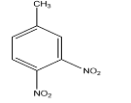
C.
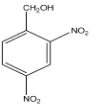
D.
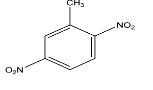




Answer
533.4k+ views
Hint: As we know that methyl ($-C{{H}_{3}}$) is an electron donating group hence being ortho – para director whereas nitro ($-N{{O}_{2}}$) is an electron withdrawing group due to which it is a meta director. On the basis of these activation sites we will add the nitro group from further nitration at these sites and get our desired product.
Complete step by step answer:
First of all let us discuss about nitration process:-
-In chemical sciences, nitration is a process of introducing the nitro group into an organic compound for various purposes. Nitration process has various industrial uses and also it is used in the production of explosives. Aside from explosives, the products formed during the process of nitration are widely used as chemical intermediates and precursors.
-Nitration of p-Nitrotoluene:-
Nitro group ($-N{{O}_{2}}$) is meta - directing since it is an electron withdrawing group therefore it will direct the incoming of another nitro group (due to nitration) at ${{2}^{\text{nd}}}$ position.
Since methyl ($-C{{H}_{3}}$) is a ortho – para directing group as it an electron donating group therefore it will direct the incoming of another nitro group (due to nitration) at ${{2}^{\text{nd}}}$ position (as ${{4}^{\text{th}}}$ position is already occupied).
From the above data we conclude that the nitro group will be added at${{2}^{\text{nd}}}$ position of the compound as shown below:-
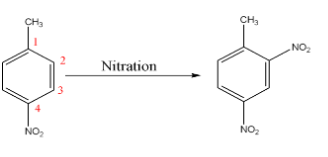
Hence the right option is (A).
Note: -If there was a compound given in the question that have both electron withdrawing and electron donating group attached with the ring and we get more than one position to add another substituent, then we will form the product according to the electron donating group as it will be the dominating factor (no matter how weak or strong it is compared to the electron withdrawing group).
Complete step by step answer:
First of all let us discuss about nitration process:-
-In chemical sciences, nitration is a process of introducing the nitro group into an organic compound for various purposes. Nitration process has various industrial uses and also it is used in the production of explosives. Aside from explosives, the products formed during the process of nitration are widely used as chemical intermediates and precursors.
-Nitration of p-Nitrotoluene:-
Nitro group ($-N{{O}_{2}}$) is meta - directing since it is an electron withdrawing group therefore it will direct the incoming of another nitro group (due to nitration) at ${{2}^{\text{nd}}}$ position.
Since methyl ($-C{{H}_{3}}$) is a ortho – para directing group as it an electron donating group therefore it will direct the incoming of another nitro group (due to nitration) at ${{2}^{\text{nd}}}$ position (as ${{4}^{\text{th}}}$ position is already occupied).
From the above data we conclude that the nitro group will be added at${{2}^{\text{nd}}}$ position of the compound as shown below:-

Hence the right option is (A).
Note: -If there was a compound given in the question that have both electron withdrawing and electron donating group attached with the ring and we get more than one position to add another substituent, then we will form the product according to the electron donating group as it will be the dominating factor (no matter how weak or strong it is compared to the electron withdrawing group).
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




