
How many POP bonds appear in cyclic metaphosphoric acid?
A. Four
B. Three
C. Two
D. One
Answer
595.2k+ views
Hint: To know the number of POP bonds, we first draw the structure of metaphosphoric acid. After drawing the compound, we have to observe P-O-P bonds in the compound.
Complete step-by-step answer:
Now, we draw the structure of cyclic metaphosphoric acid.
The cyclic metaphosphoric acid consists of three phosphorus atoms, nine oxygen atoms and three hydrogen atoms. There are two types of bond in the cyclic metaphosphoric acid, that is, single bond and double bond. There are three double bonds and nine single bonds in the compound.
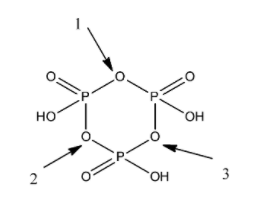
From the diagram of cyclic metaphosphoric acid, we find that there are three P-O-P bonds in the compound.
So, option B is correct.
Additional Information:
1. Chemical bond: The force of attraction between atoms results in a chemical bond. The chemical bond may be of different types, ionic bond, covalent bond and coordinate bond. The sharing of electrons between atoms results in covalent bond, the transfer of electrons results in ionic bond.
2. Metaphosphoric acid: Fujita and Iwatake suggested that approximate 2% concentration of metaphosphoric acid protects vitamin C in solution against the atmospheric oxidation in the presence of copper and also protects oxidation in the presence of trichloroacetic acid. The deprotonation of one of two OH groups gives the monovalent inorganic anion, that is, phosphonate.
Note: Students must correctly draw the structure of cyclic metaphosphoric acid. If the structure is drawn incorrect, the number of P-O-P bonds will differ and we are unable to choose the correct option.
Complete step-by-step answer:
Now, we draw the structure of cyclic metaphosphoric acid.
The cyclic metaphosphoric acid consists of three phosphorus atoms, nine oxygen atoms and three hydrogen atoms. There are two types of bond in the cyclic metaphosphoric acid, that is, single bond and double bond. There are three double bonds and nine single bonds in the compound.

From the diagram of cyclic metaphosphoric acid, we find that there are three P-O-P bonds in the compound.
So, option B is correct.
Additional Information:
1. Chemical bond: The force of attraction between atoms results in a chemical bond. The chemical bond may be of different types, ionic bond, covalent bond and coordinate bond. The sharing of electrons between atoms results in covalent bond, the transfer of electrons results in ionic bond.
2. Metaphosphoric acid: Fujita and Iwatake suggested that approximate 2% concentration of metaphosphoric acid protects vitamin C in solution against the atmospheric oxidation in the presence of copper and also protects oxidation in the presence of trichloroacetic acid. The deprotonation of one of two OH groups gives the monovalent inorganic anion, that is, phosphonate.
Note: Students must correctly draw the structure of cyclic metaphosphoric acid. If the structure is drawn incorrect, the number of P-O-P bonds will differ and we are unable to choose the correct option.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




