
Predict the products formed when cyclohexanecarbaldehyde reacts with following reagents
(i) PhMgBr and then ${{H}_{3}}{{O}^{+}}$
(ii) Tollen reagent
(iii) Semicarbazide and weak acid.
Answer
566.7k+ views
Hint: We are asked to find the products formed when the compound cyclohexanecarbaldehyde is treated with certain reagents. Cyclohexanecarbaldehyde is a carbonyl compound and Phenylmagnesium bromide (PhMgBr or ${{C}_{6}}{{H}_{5}}MgBr$) is a strong base as well as a strong nucleophile and it can abstract even mildly acidic protons.
Complete answer:
(i) PhMgBr and then ${{H}_{3}}{{O}^{+}}$
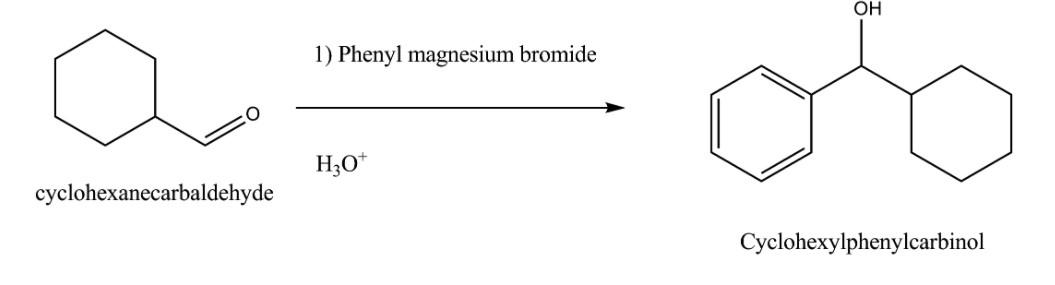
PhMgBr is often added to carbonyl compounds such as aldehydes and ketones and it attacks the electrophilic carbonyl carbon atom to give an alkoxide intermediate and the subsequent protonation will yield an alcohol as product.
The reaction of cyclohexanecarbaldehyde with Phenylmagnesium bromide followed by the acid hydrolysis (${{H}_{3}}{{O}^{+}}$) will give us alcohol as product. The alcohol formed is Cyclohexyl Phenyl Carbinol and the reaction can be represented as follows
(ii) Tollen reagent
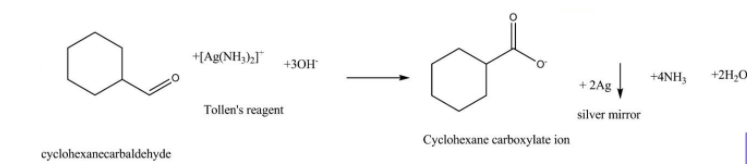
The compound Cyclohexanecarbaldehyde will react with tollen's reagent and reduce it to silver (Ag) and oxidises itself to form cyclohexane carboxylate ion.Or in other words the oxidation of cyclohexanecarbaldehyde with Tollen’s reagent gives cyclohexane carboxylate ion and the corresponding reaction can be represented as follows
(iii) Semicarbazide and weak acid.
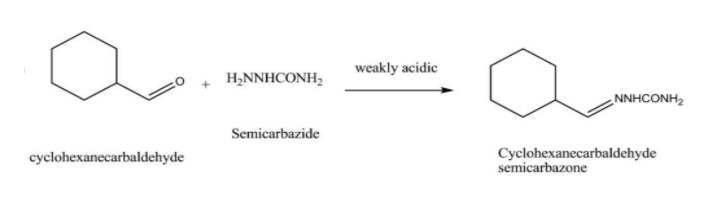
When cyclohexanecarbaldehyde is treated with semicarbazide and weak acid , the product formed is cyclohexanecarbaldehyde semicarbazone and the reaction can be represented as follows
Note: The other major reactions which include cyclohexanecarbaldehyde involves its treatment with excess ethanol and acid gives the product Cyclohexanecarbaldehyde diethyl acetal. When cyclohexanecarbaldehyde is treated with Zinc amalgam and concentrated hydrochloric acid the −CHO group is reduced to $-C{{H}_{3}}$ group and the product formed will be 1-Methyl cyclohexane.
Complete answer:
(i) PhMgBr and then ${{H}_{3}}{{O}^{+}}$
PhMgBr is often added to carbonyl compounds such as aldehydes and ketones and it attacks the electrophilic carbonyl carbon atom to give an alkoxide intermediate and the subsequent protonation will yield an alcohol as product.
The reaction of cyclohexanecarbaldehyde with Phenylmagnesium bromide followed by the acid hydrolysis (${{H}_{3}}{{O}^{+}}$) will give us alcohol as product. The alcohol formed is Cyclohexyl Phenyl Carbinol and the reaction can be represented as follows

(ii) Tollen reagent
The compound Cyclohexanecarbaldehyde will react with tollen's reagent and reduce it to silver (Ag) and oxidises itself to form cyclohexane carboxylate ion.Or in other words the oxidation of cyclohexanecarbaldehyde with Tollen’s reagent gives cyclohexane carboxylate ion and the corresponding reaction can be represented as follows

(iii) Semicarbazide and weak acid.
When cyclohexanecarbaldehyde is treated with semicarbazide and weak acid , the product formed is cyclohexanecarbaldehyde semicarbazone and the reaction can be represented as follows

Note: The other major reactions which include cyclohexanecarbaldehyde involves its treatment with excess ethanol and acid gives the product Cyclohexanecarbaldehyde diethyl acetal. When cyclohexanecarbaldehyde is treated with Zinc amalgam and concentrated hydrochloric acid the −CHO group is reduced to $-C{{H}_{3}}$ group and the product formed will be 1-Methyl cyclohexane.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




