
Preparation of terylene a polyester or Dacron:
Answer
563.7k+ views
Hint:Terylene is a polyester also called Dacron. It is formed by condensation of ethylene glycol and p-terephthalic acid ($1,4$-benzene dicarboxylic acid). So there will be condensation polymerization.
Complete answer:
First, we will understand the term ‘Polymerization’. It is a process in which very small molecules termed ‘monomers’ combine to produce a very large network structure called a polymer. Simply, it is a process to create polymers.
Now we will understand the preparation of terylene, a polyester or Dacron. It is prepared by the condensation of ethylene glycol and p-terephthalic acid ($1,4$-benzene dicarboxylic acid) in presence of a weak base like calcium acetate.
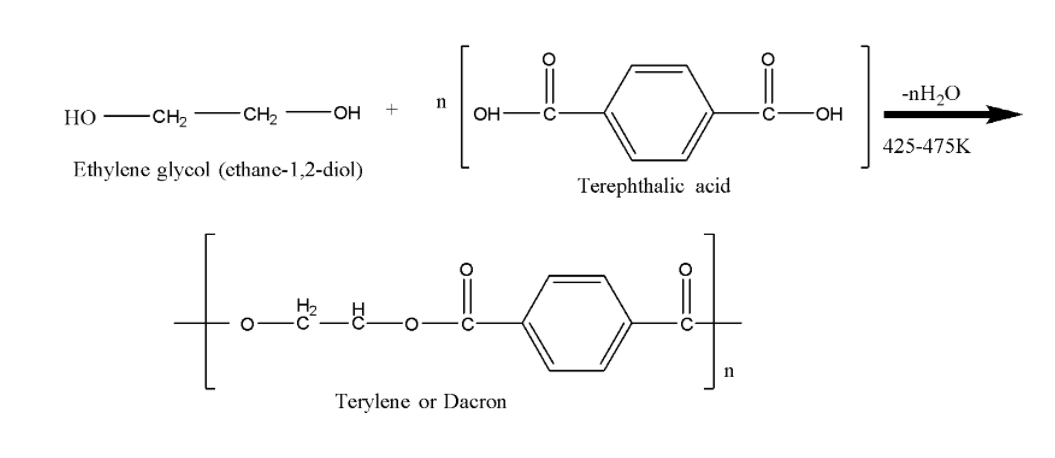
We can observe that there are two monomers ethylene glycol and p-terephthalic acid ($1,4$-benzene dicarboxylic acid) that are condensed and after the removal of water we obtain Terylene or dacron.
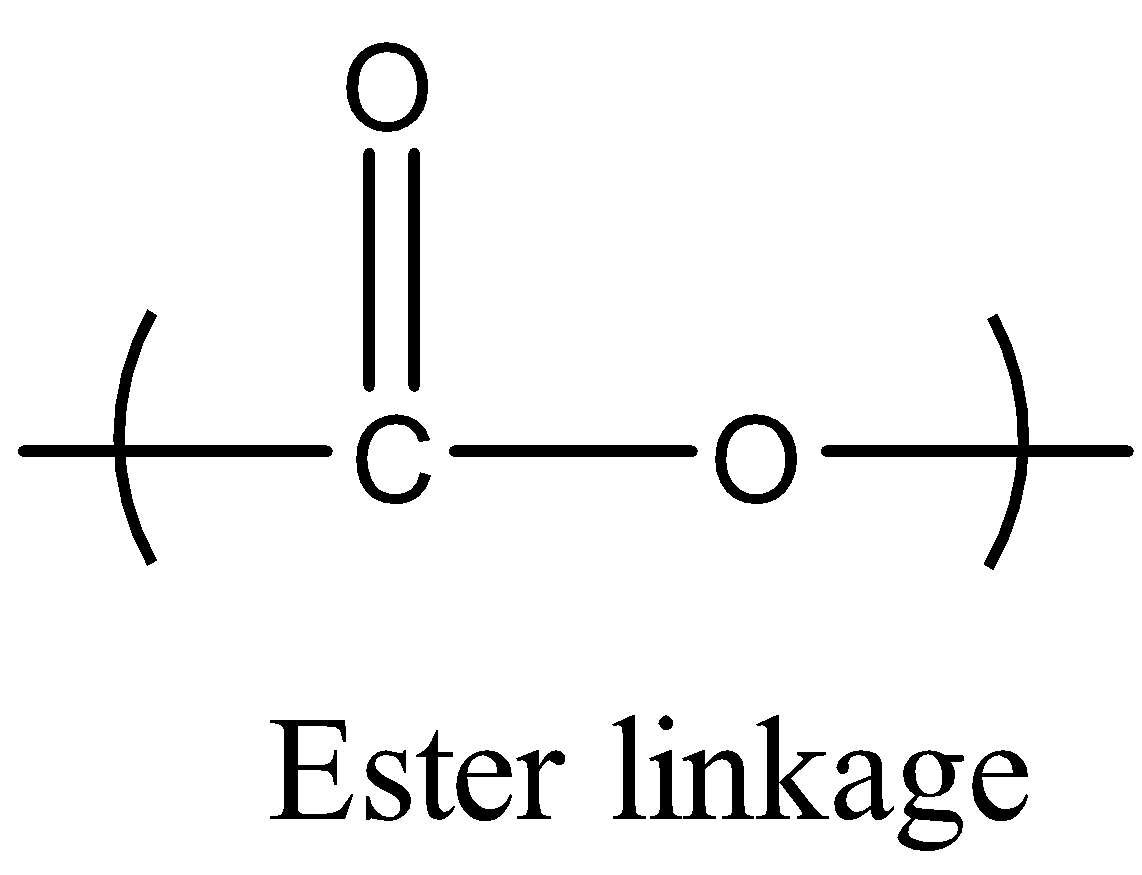
We can also observe one linkage in the obtained polyester Terylene or Dacron called an ester linkage. The ester linkage is shown below.There are many important uses and properties of the polyester Terylene or Dacron.
Additional Details:
1.It has high tensile strength.
2.It has low moisture absorbing power and dries up very quickly.
3.It is resistant to chemical or biological substances.
4.Its melt can be spun into fibers and clothes retain creases.
5.Its fiber is strong, durable, and flexible.
Note:
Due to low moisture absorbing power, it is used for making wash and wear clothes.
It is used as a blend of cotton and wool to increase their resistance to wear and tear.
Dacron and Teflon tubes are a good substitute for human blood vessels and used in heart bypass surgical operations.
Complete answer:
First, we will understand the term ‘Polymerization’. It is a process in which very small molecules termed ‘monomers’ combine to produce a very large network structure called a polymer. Simply, it is a process to create polymers.
Now we will understand the preparation of terylene, a polyester or Dacron. It is prepared by the condensation of ethylene glycol and p-terephthalic acid ($1,4$-benzene dicarboxylic acid) in presence of a weak base like calcium acetate.

We can observe that there are two monomers ethylene glycol and p-terephthalic acid ($1,4$-benzene dicarboxylic acid) that are condensed and after the removal of water we obtain Terylene or dacron.
We can also observe one linkage in the obtained polyester Terylene or Dacron called an ester linkage. The ester linkage is shown below.There are many important uses and properties of the polyester Terylene or Dacron.

Additional Details:
1.It has high tensile strength.
2.It has low moisture absorbing power and dries up very quickly.
3.It is resistant to chemical or biological substances.
4.Its melt can be spun into fibers and clothes retain creases.
5.Its fiber is strong, durable, and flexible.
Note:
Due to low moisture absorbing power, it is used for making wash and wear clothes.
It is used as a blend of cotton and wool to increase their resistance to wear and tear.
Dacron and Teflon tubes are a good substitute for human blood vessels and used in heart bypass surgical operations.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




