
Prepare 2-iodo-2-methylpropane from 2-chloro-2-methylpropane.
Answer
535.2k+ views
Hint: The given reactants and forming product both are alkyl halides in nature and alkyl halides are those compounds in which halogen is present with alkyl groups where halogens are iodine, chlorine, bromine etc.
Complete step by step answer: The reaction can be done under the reaction named Finkelstein reaction which kept in the category of substitution nucleophilic reaction and substitution nuclear is of two types known as\[S{{N}^{1}}\] and \[S{{N}^{2}}\] reactions i.e. Substitution nucleophilic unimolecular reaction and substitution nucleophilic bimolecular reaction.
The Finkelstein reaction is kept under the category of \[S{{N}^{2}}\]reaction which generally involves the exchange of halogen atom and this reaction is named after the name of German scientist who discovered it i.e. Hans Finkelstein.
It is basically an organic reaction in which an alkyl halide exchange into another alkyl halide through a reaction wherein the metal halide salt i.e. sodium iodide is used and reaction takes place at an equilibrium process in the presence of acetone. Reaction between 2-iodo-2-methylpropane from 2-chloro-2-methyl propane can be shown as:
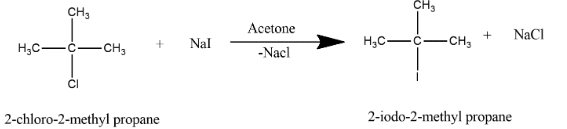
The mechanism of the Finkelstein reaction is single-step reaction with stereochemistry inversion and the reaction involves the process of an alkyl bromide or an alkyl chloride into an alkyl iodide which is treated with a sodium iodide solution in acetone.
Note: Only sodium iodide solution is used in this reaction the reason behind this is sodium iodide is soluble in the acetone while the other solutions like sodium bromide and sodium chloride are not soluble in the acetone.
Complete step by step answer: The reaction can be done under the reaction named Finkelstein reaction which kept in the category of substitution nucleophilic reaction and substitution nuclear is of two types known as\[S{{N}^{1}}\] and \[S{{N}^{2}}\] reactions i.e. Substitution nucleophilic unimolecular reaction and substitution nucleophilic bimolecular reaction.
The Finkelstein reaction is kept under the category of \[S{{N}^{2}}\]reaction which generally involves the exchange of halogen atom and this reaction is named after the name of German scientist who discovered it i.e. Hans Finkelstein.
It is basically an organic reaction in which an alkyl halide exchange into another alkyl halide through a reaction wherein the metal halide salt i.e. sodium iodide is used and reaction takes place at an equilibrium process in the presence of acetone. Reaction between 2-iodo-2-methylpropane from 2-chloro-2-methyl propane can be shown as:

The mechanism of the Finkelstein reaction is single-step reaction with stereochemistry inversion and the reaction involves the process of an alkyl bromide or an alkyl chloride into an alkyl iodide which is treated with a sodium iodide solution in acetone.
Note: Only sodium iodide solution is used in this reaction the reason behind this is sodium iodide is soluble in the acetone while the other solutions like sodium bromide and sodium chloride are not soluble in the acetone.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




