
How do you prepare phenol from benzene?
Answer
552k+ views
Hint: Conversion of benzene to phenol means introduction of hydroxyl group in the benzene ring. This introduction can be done with various processes and mechanisms. The Majority of the processes involved in this conversion are two steps or multi-steps processes.
Complete step-by-step answer:Phenol can be prepared from benzene in many ways. Here we will discuss two major ways through which the reaction can proceed.
1. From chlorobenzene: Benzene undergo halogenation when it is treated with chlorine in presence of Lewis catalyst such as anhydrous $FeC{{l}_{3}}$or $AlC{{l}_{3}}$to yield chlorobenzene. The mechanism involves generation of an electrophile. Electrophile then attacks the benzene to generate carbocation. Finally, removal of proton yields the chlorobenzene.
The final reaction is as follows:
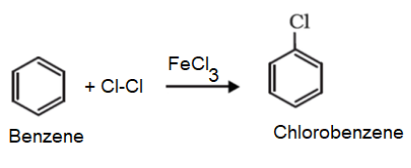
Chlorobenzene so formed can be converted to phenol as follows
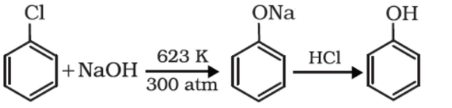
Chlorobenzene on heating with 10 percent aqueous sodium hydroxide solution at about $623K$under $300atm$ yields sodium phenoxide which when further treated with dilute hydrochloric acid yields phenol. This is termed as Dow’s process.
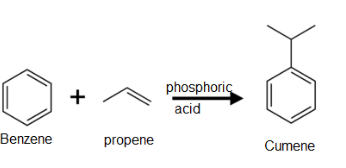
2. From Cumene
Phenol is prepared commercially from cumene. Cumene is prepared by Friedel craft alkylation of benzene with propene in the presence of phosphoric acid at 523K.
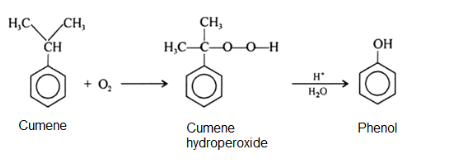
Cumene is oxidized in the presence of air to cumene hydroperoxide which upon subsequent hydrolysis with sulfuric acid gives phenol and propanone
Note: It should be noted that optimum temperature and proper catalyst needs to be used while performing the reactions. Also, there are various other methods through which the phenol can be prepared, but the most commercial method is the preparation with the help of cumene.
Complete step-by-step answer:Phenol can be prepared from benzene in many ways. Here we will discuss two major ways through which the reaction can proceed.
1. From chlorobenzene: Benzene undergo halogenation when it is treated with chlorine in presence of Lewis catalyst such as anhydrous $FeC{{l}_{3}}$or $AlC{{l}_{3}}$to yield chlorobenzene. The mechanism involves generation of an electrophile. Electrophile then attacks the benzene to generate carbocation. Finally, removal of proton yields the chlorobenzene.
The final reaction is as follows:

Chlorobenzene so formed can be converted to phenol as follows
Chlorobenzene on heating with 10 percent aqueous sodium hydroxide solution at about $623K$under $300atm$ yields sodium phenoxide which when further treated with dilute hydrochloric acid yields phenol. This is termed as Dow’s process.

2. From Cumene
Phenol is prepared commercially from cumene. Cumene is prepared by Friedel craft alkylation of benzene with propene in the presence of phosphoric acid at 523K.

Cumene is oxidized in the presence of air to cumene hydroperoxide which upon subsequent hydrolysis with sulfuric acid gives phenol and propanone

Note: It should be noted that optimum temperature and proper catalyst needs to be used while performing the reactions. Also, there are various other methods through which the phenol can be prepared, but the most commercial method is the preparation with the help of cumene.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




