
How will you prepare: propiophenone from propane nitrile.
Answer
587.1k+ views
Hint: The chemical reactions which involve organic compounds are known as organic reactions. Generally, the main motive for the occurrence of the chemical reactions is to form more stable compounds. It is to be remembered always that stronger elements have the power to displace the weaker ones.
Complete step by step answer:
Propane nitrile is an organic compound with the chemical formula of $C{H_3}C{H_2}CN$. It is also known as ethyl cyanide. For the conversion of propane nitrile to propiophenone, firstly acid hydrolysis of propane nitrile is done. Acid hydrolysis is a process in which a protic acid is used to catalyze the cleavage of a chemical bond by a nucleophilic substitution reaction with the addition of water $\left( {{H_2}O} \right)$. This step can be written as:
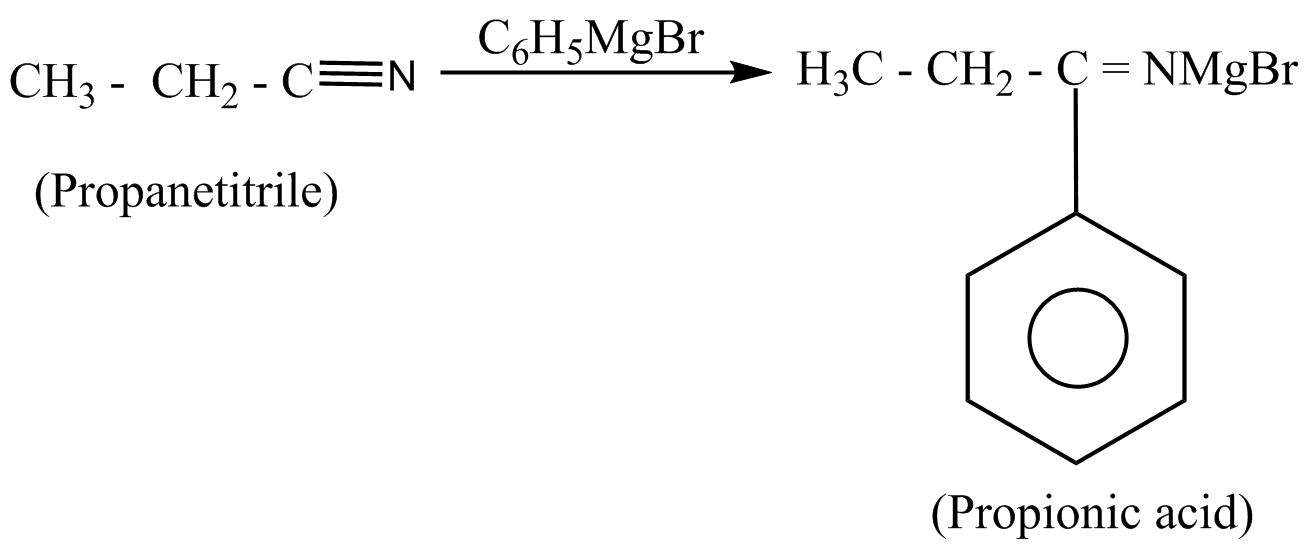
Then, the propionic acid undergoes the nucleophilic substitution reaction by using the Grignard’s reagent. In nucleophilic substitution reaction, a leaving group is replaced by an electron rich compound which is called a nucleophile. This reaction can be written as:
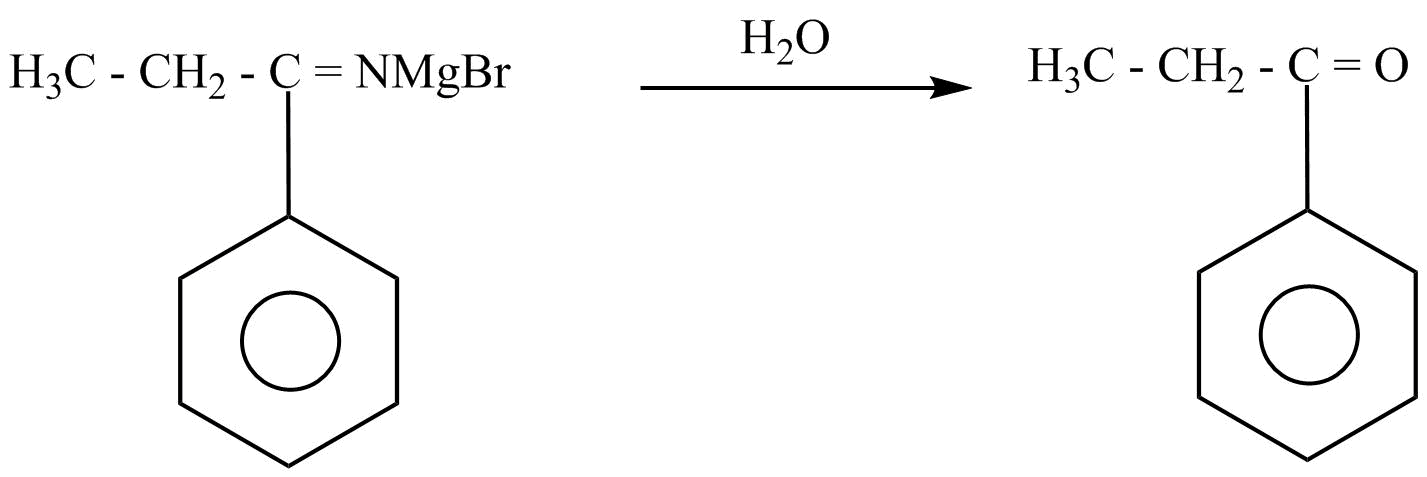
Hence, propane nitrile is converted into propiophenone.
Note:
Propionitrile, also known as ethyl cyanide and propanenitrile, is an organic compound with the formula $CH_3CH_2CN$. It is a simple aliphatic nitrile. The compound is a colourless, water-soluble liquid. It is used as a solvent and a precursor to other organic compounds.
Complete step by step answer:
Propane nitrile is an organic compound with the chemical formula of $C{H_3}C{H_2}CN$. It is also known as ethyl cyanide. For the conversion of propane nitrile to propiophenone, firstly acid hydrolysis of propane nitrile is done. Acid hydrolysis is a process in which a protic acid is used to catalyze the cleavage of a chemical bond by a nucleophilic substitution reaction with the addition of water $\left( {{H_2}O} \right)$. This step can be written as:

Then, the propionic acid undergoes the nucleophilic substitution reaction by using the Grignard’s reagent. In nucleophilic substitution reaction, a leaving group is replaced by an electron rich compound which is called a nucleophile. This reaction can be written as:

Hence, propane nitrile is converted into propiophenone.
Note:
Propionitrile, also known as ethyl cyanide and propanenitrile, is an organic compound with the formula $CH_3CH_2CN$. It is a simple aliphatic nitrile. The compound is a colourless, water-soluble liquid. It is used as a solvent and a precursor to other organic compounds.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




