
What product is formed in the treatment of cyclopentene with the bromine water?
(A) trans-3-bromocyclopentanol
(B) cis-2-bromocyclopentanol
(C) trans-2-bromocyclopentanol
(D) cis-3-bromocyclopentanol
Answer
567k+ views
Hint: In order to answer the question, we must have an idea about different organic reactions that will be taking place. So, it will become easier to answer what will be the product formed when cyclopentene is treated with the bromine water. This reaction will be following Markovnikov’s rule.
Complete Solution :
The treatment of the cyclopentene with the bromine water is an electrophilic substitution reaction.
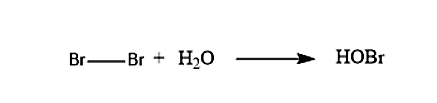
In the first step when the Bromine molecule and the water molecule reacts with each other, it will lead to the formation of the Bromohydrins. The reaction of the formation of the Bromohydrin is given below:
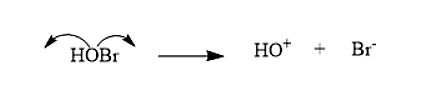
- The bromohydrin will break in such a way that the Halide is the negative species, i.e. \[B{r^ - }\], while the hydrin is the positive species i.e. \[H{O^ + }\]
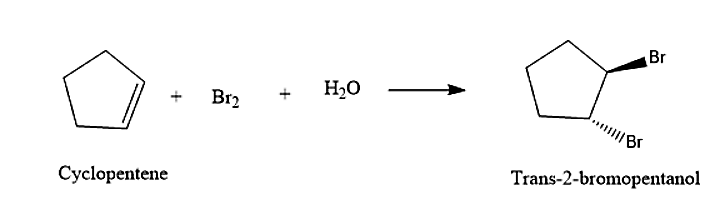
- The cyclopentene will react with the formed bromohydrin to give Trans-2-bromopentanol. The and will attack across the double bond. This reaction will be following Markovnikov’s rule. Formation of the Trans-2-bromopentanol is given below:
So, the correct answer is “Option C”.
Note: - Bromine water is usually used to test whether the given compound is saturated or unsaturated.
- Organic compounds such as alkene, phenols and aniline will undergo reaction with bromine water readily.
- When the colour of the bromine water changes, it will indicate the presence of an unsaturated compound.
Complete Solution :
The treatment of the cyclopentene with the bromine water is an electrophilic substitution reaction.
In the first step when the Bromine molecule and the water molecule reacts with each other, it will lead to the formation of the Bromohydrins. The reaction of the formation of the Bromohydrin is given below:

- The bromohydrin will break in such a way that the Halide is the negative species, i.e. \[B{r^ - }\], while the hydrin is the positive species i.e. \[H{O^ + }\]

- The cyclopentene will react with the formed bromohydrin to give Trans-2-bromopentanol. The and will attack across the double bond. This reaction will be following Markovnikov’s rule. Formation of the Trans-2-bromopentanol is given below:

So, the correct answer is “Option C”.
Note: - Bromine water is usually used to test whether the given compound is saturated or unsaturated.
- Organic compounds such as alkene, phenols and aniline will undergo reaction with bromine water readily.
- When the colour of the bromine water changes, it will indicate the presence of an unsaturated compound.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




