
Propanoic acid occurs naturally as a result of the bacterial fermentation of milk and is partly responsible for the flavour of Swiss cheese.
Which starting materials can be used to produce propanoic acid?
$1.$${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{OH}}$
$2.$${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CHO}}$
$3.$${\text{C}}{{\text{H}}_{\text{3}}}{\text{C}}{{\text{H}}_{\text{2}}}{\text{CN}}$
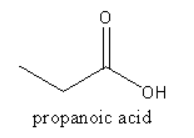
A. $1,2$ and $3$ are correct.
B. $1$ and $2$ only are correct.
B. $2$ and $3$ only are correct.
D. $1$ only is correct.

Answer
569.7k+ views
Hint:The given reactant contains alcohol, aldehyde, and nitrile, functional group. We will convert them into carboxylic acid by using oxidizing agents. Oxidizing agents cause oxidation hence converts alcohol, aldehyde, and nitrile functional groups into a carboxylic acid.
Complete answer:
We have to prepare propanoic acid from alcohol, aldehyde, and nitrile functional groups. The conversion of alcohol, aldehyde and nitrile into acid is known as oxidation. For oxidation, strong oxidizing agents are required.
The oxidation reaction are shown as follows:
1. Potassium permanganate converts alcohol into acid.
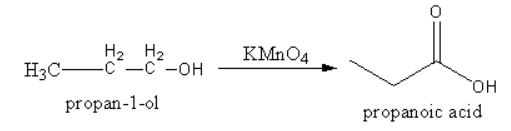
2. Chromic acid converts aldehyde into acid.

3. By simple hydrolysis nitrile can be converted into acid.

So, propanol, propionaldehyde, propionitrile all three can be converted into acid.
Therefore, option (A) $1,2$ and $3$ are correct, is correct.
Additional information:
The substances which cause oxidation are known as oxidation agents. . In place of chromic acid and potassium permanganate some other oxidizing agent can be used such as a solution of chromic acid with sulphuric acid ${\text{Cr}}{{\text{O}}_3}{\text{/}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_4}$which is known as Jones reagent. A solution of ferrous sulphate with hydrogen peroxide ${\text{FeS}}{{\text{O}}_{\text{4}}}{\text{/}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$ is known as Fenton reagent. Hydrogen peroxide itself also works as an oxidizing agent. The Swarn oxidation and PCC are used to convert primary alcohol into acid.
Note:
Chromic acid and potassium permanganate convert primary, secondary alcohol, and ketone into a carboxylic acid. Chromic acid and potassium permanganate both first convert alcohol into aldehyde and then into a carboxylic acid. Both are strong oxidizing agents. Potassium permanganate works in an alkaline medium. Partial hydrolysis of the nitrile gives the amides and complete hydrolysis gives carboxylic acid.
Complete answer:
We have to prepare propanoic acid from alcohol, aldehyde, and nitrile functional groups. The conversion of alcohol, aldehyde and nitrile into acid is known as oxidation. For oxidation, strong oxidizing agents are required.
The oxidation reaction are shown as follows:
1. Potassium permanganate converts alcohol into acid.

2. Chromic acid converts aldehyde into acid.

3. By simple hydrolysis nitrile can be converted into acid.

So, propanol, propionaldehyde, propionitrile all three can be converted into acid.
Therefore, option (A) $1,2$ and $3$ are correct, is correct.
Additional information:
The substances which cause oxidation are known as oxidation agents. . In place of chromic acid and potassium permanganate some other oxidizing agent can be used such as a solution of chromic acid with sulphuric acid ${\text{Cr}}{{\text{O}}_3}{\text{/}}{{\text{H}}_{\text{2}}}{\text{S}}{{\text{O}}_4}$which is known as Jones reagent. A solution of ferrous sulphate with hydrogen peroxide ${\text{FeS}}{{\text{O}}_{\text{4}}}{\text{/}}{{\text{H}}_{\text{2}}}{{\text{O}}_{\text{2}}}$ is known as Fenton reagent. Hydrogen peroxide itself also works as an oxidizing agent. The Swarn oxidation and PCC are used to convert primary alcohol into acid.
Note:
Chromic acid and potassium permanganate convert primary, secondary alcohol, and ketone into a carboxylic acid. Chromic acid and potassium permanganate both first convert alcohol into aldehyde and then into a carboxylic acid. Both are strong oxidizing agents. Potassium permanganate works in an alkaline medium. Partial hydrolysis of the nitrile gives the amides and complete hydrolysis gives carboxylic acid.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




