
How is propanone converted into (i) \[propan - 2 - ol\] (ii) \[2 - methylpropan - 2 - ol\]
Answer
564.9k+ views
Hint:Propanone is an organic compound with a chemical formula ${C_3}{H_6}O$. It is the simplest and smallest member of the ketone family. Its common name is acetone. Acetone is a colorless, highly volatile, and flammable liquid. It has a very pungent odour.
Complete answer:
(i) Propanone to \[propan - 2 - ol\]
Conversion of Propanone to \[propan - 2 - ol\] is done by reduction. Propanone is a carbonyl compound with functional groups.
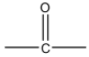
We have to convert propanone with $CO$ functional group to alcohol with $ - OH$ functional group. When we reduce carbonyl compounds in the presence of a reducing agent, they get converted to alcohol. So we will treat propanone with a reducing agent like lithium aluminum hydride $(LiAl{H_4})$ and Sodium borohydride $(NaB{H_4})$. On treatment with $(LiAl{H_4})$ propanone will be reduced to \[propan - 2 - ol\] . It is also called isopropyl alcohol. The reaction will be:
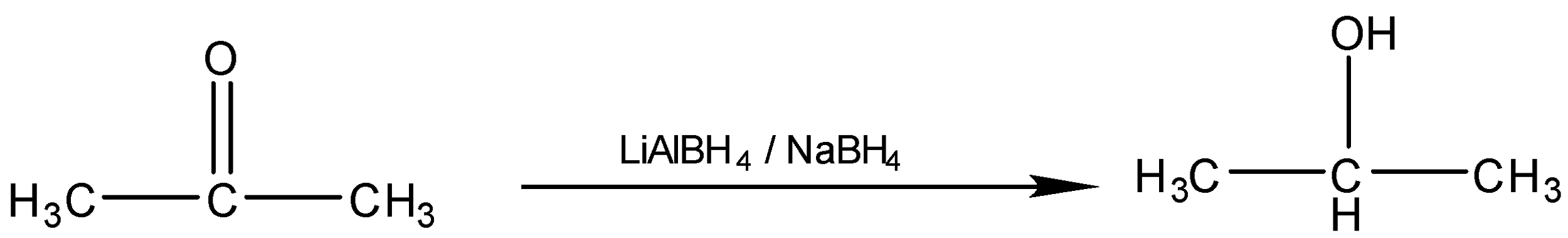
(ii) Propanone to \[2 - methylpropan - 2 - ol\]
The conversion of Propanone to \[2 - methylpropan - 2 - ol\]is a two-step process. So First we will treat the Ketone with Grignard reagent i.e. $R - MgX$ where $R$ is the methyl group and $X$ is the halogen. After that, we have to do the acidic hydrolysis of the compound. When we react Propanone with $C{H_3} - MgBr$, the reagent will break into two parts $\mathop {C{H_3}}\limits^ - $ and $\mathop {MgBr}\limits^ + $, so oxygen being the electronegative atom will attract the shared electron pairs towards itself creating polarity in the bond. The $\mathop {C{H_3}}\limits^ - $ electrophile will attack there and forms the bond with the carbonyl carbon and $\mathop {MgBr}\limits^ + $ will bond with oxygen forming an intermediate. In the second step, the acidic hydrolysis of the intermediate is done to replace the $O - MgBr$ group with $ - OH$. The reaction of the conversion will be:
Note:
\[propan - 2 - ol\] or isopropyl alcohol is a colorless, flammable organic compound that has a very strong odor. It is used in the manufacturing of antiseptics, disinfectants, and a wide variety of household chemicals.
Complete answer:
(i) Propanone to \[propan - 2 - ol\]
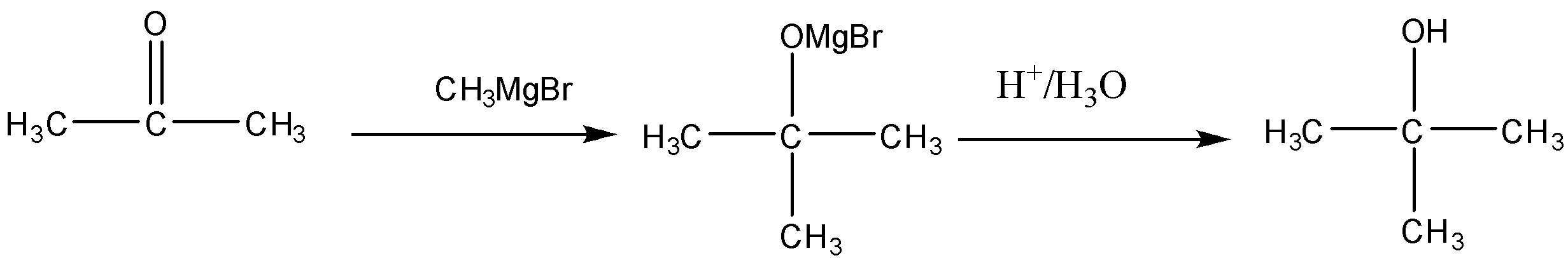
Conversion of Propanone to \[propan - 2 - ol\] is done by reduction. Propanone is a carbonyl compound with functional groups.

We have to convert propanone with $CO$ functional group to alcohol with $ - OH$ functional group. When we reduce carbonyl compounds in the presence of a reducing agent, they get converted to alcohol. So we will treat propanone with a reducing agent like lithium aluminum hydride $(LiAl{H_4})$ and Sodium borohydride $(NaB{H_4})$. On treatment with $(LiAl{H_4})$ propanone will be reduced to \[propan - 2 - ol\] . It is also called isopropyl alcohol. The reaction will be:
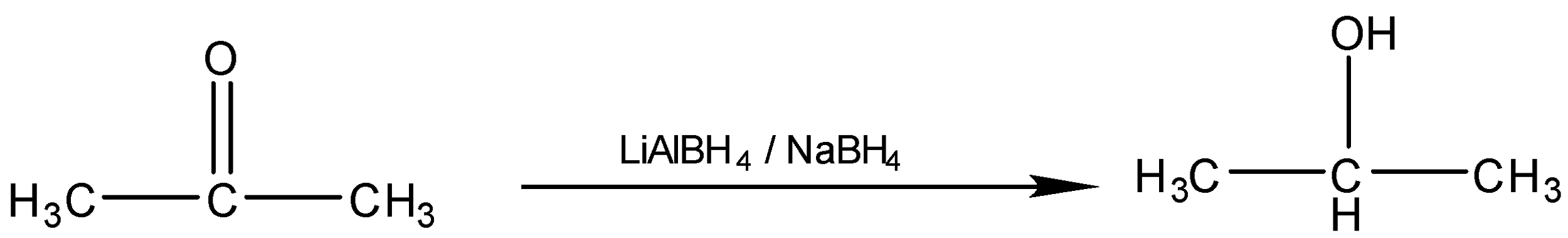
(ii) Propanone to \[2 - methylpropan - 2 - ol\]
The conversion of Propanone to \[2 - methylpropan - 2 - ol\]is a two-step process. So First we will treat the Ketone with Grignard reagent i.e. $R - MgX$ where $R$ is the methyl group and $X$ is the halogen. After that, we have to do the acidic hydrolysis of the compound. When we react Propanone with $C{H_3} - MgBr$, the reagent will break into two parts $\mathop {C{H_3}}\limits^ - $ and $\mathop {MgBr}\limits^ + $, so oxygen being the electronegative atom will attract the shared electron pairs towards itself creating polarity in the bond. The $\mathop {C{H_3}}\limits^ - $ electrophile will attack there and forms the bond with the carbonyl carbon and $\mathop {MgBr}\limits^ + $ will bond with oxygen forming an intermediate. In the second step, the acidic hydrolysis of the intermediate is done to replace the $O - MgBr$ group with $ - OH$. The reaction of the conversion will be:

Note:
\[propan - 2 - ol\] or isopropyl alcohol is a colorless, flammable organic compound that has a very strong odor. It is used in the manufacturing of antiseptics, disinfectants, and a wide variety of household chemicals.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




