
How to prove that naphthalene is an aromatic compound by using Huckel’s rule?
Answer
558.6k+ views
Hint: To determine the answer to this question we should know the structure of the naphthalene and we should also know Huckel’s rule. The naphthalene is a bicyclic compound having two benzene rings joint at one-two positions. Huckel’s rule is used to determine that the given cyclic compound is aromatic or antiaromatic. Huckel gave two different formulas for aromatic and antiaromatic.
Complete step by step answer:
According to Huckel’s rule, the carbo-cyclic or heterocyclic compound having ${{4n\pi }}$ delocalizing electrons or pi electrons are anti-aromatic and the carbo-cyclic or heterocyclic compound having ${{4n\pi }}\, + 2$ delocalizing electrons or pi electrons are aromatic. The structure of the naphthalene is as follows:
- Aromatic compound: ${{4n\pi }}\, + 2$ delocalised electrons
- Anti-aromatic compound: ${{4n\pi }}$ delocalised electrons
Where, n is a whole number that is used to satisfy Huckel’s rule.
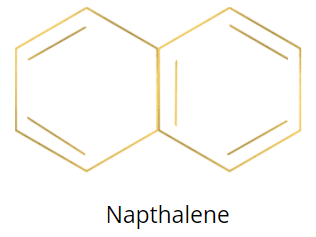
Naphthalene has five pi bonds (two in the first ring and three in the second ring). Each pi bond has two electrons, so naphthalene has a total of ten pi delocalizing electrons.
We arbitrarily choose ${\text{n = 2}}$.
When we substitute ${\text{n = 2}}$ in ${{4n\pi }}$ formula we get,
$ \Rightarrow {\text{4}} \times 2{{\pi }}$
$ \Rightarrow {{8\pi }}$ delocalised electrons.
When we substitute ${\text{n = 2}}$ in ${{4n\pi }}\, + 2$ formula we get,
$ \Rightarrow {\text{4}} \times 2{{\pi }} + 2$
$ \Rightarrow {\text{10}}\,{{\pi }}$
${{4n\pi }}\, + 2$ formula is giving ten pi electrons and our naphthalene also has ten pin delocalised electrons it means the naphthalene is aromatic.
We can calculate the ${\text{n = 2}}$ as follows:
Naphthalene has ten pi electrons so, on substituting ten pi electrons in ${{4n\pi }}\, + 2$ formula,
$ \Rightarrow {{4n\pi }} + 2\, = {\text{10}}\,{{\pi }}$
$ \Rightarrow {{4n\pi }}\, = {\text{10}}\,{{\pi }} - {\text{2}}$
$ \Rightarrow {{4n\pi }}\, = 8\,{{\pi }}$
$ \Rightarrow {\text{n}}\, = 8\,{{\pi /4}}\,{{\pi }}$
$ \Rightarrow {\text{n}}\, = 2$
As we are getting a whole number from, ${{4n\pi }}\, + 2$ formula for naphthalene, so naphthalene is aromatic.
Therefore, the naphthalene is an aromatic compound according to Huckel’s rule because it has ${{4n \pi }}\, + 2$ delocalised electrons.
Note: Pi bonds are known as delocalized bonds. Pi bonds cause the resonance. Sigma bond cannot delocalize. The aromatic compounds are planer and have all ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridized carbon atoms. The aromatic compounds have complete conjugation. All cyclic compounds with ten pi electrons will always be aromatic if they are planer. The aromatic compounds are more stable than the anti-aromatic.
Complete step by step answer:
According to Huckel’s rule, the carbo-cyclic or heterocyclic compound having ${{4n\pi }}$ delocalizing electrons or pi electrons are anti-aromatic and the carbo-cyclic or heterocyclic compound having ${{4n\pi }}\, + 2$ delocalizing electrons or pi electrons are aromatic. The structure of the naphthalene is as follows:
- Aromatic compound: ${{4n\pi }}\, + 2$ delocalised electrons
- Anti-aromatic compound: ${{4n\pi }}$ delocalised electrons
Where, n is a whole number that is used to satisfy Huckel’s rule.

Naphthalene has five pi bonds (two in the first ring and three in the second ring). Each pi bond has two electrons, so naphthalene has a total of ten pi delocalizing electrons.
We arbitrarily choose ${\text{n = 2}}$.
When we substitute ${\text{n = 2}}$ in ${{4n\pi }}$ formula we get,
$ \Rightarrow {\text{4}} \times 2{{\pi }}$
$ \Rightarrow {{8\pi }}$ delocalised electrons.
When we substitute ${\text{n = 2}}$ in ${{4n\pi }}\, + 2$ formula we get,
$ \Rightarrow {\text{4}} \times 2{{\pi }} + 2$
$ \Rightarrow {\text{10}}\,{{\pi }}$
${{4n\pi }}\, + 2$ formula is giving ten pi electrons and our naphthalene also has ten pin delocalised electrons it means the naphthalene is aromatic.
We can calculate the ${\text{n = 2}}$ as follows:
Naphthalene has ten pi electrons so, on substituting ten pi electrons in ${{4n\pi }}\, + 2$ formula,
$ \Rightarrow {{4n\pi }} + 2\, = {\text{10}}\,{{\pi }}$
$ \Rightarrow {{4n\pi }}\, = {\text{10}}\,{{\pi }} - {\text{2}}$
$ \Rightarrow {{4n\pi }}\, = 8\,{{\pi }}$
$ \Rightarrow {\text{n}}\, = 8\,{{\pi /4}}\,{{\pi }}$
$ \Rightarrow {\text{n}}\, = 2$
As we are getting a whole number from, ${{4n\pi }}\, + 2$ formula for naphthalene, so naphthalene is aromatic.
Therefore, the naphthalene is an aromatic compound according to Huckel’s rule because it has ${{4n \pi }}\, + 2$ delocalised electrons.
Note: Pi bonds are known as delocalized bonds. Pi bonds cause the resonance. Sigma bond cannot delocalize. The aromatic compounds are planer and have all ${\text{s}}{{\text{p}}^{\text{2}}}$ hybridized carbon atoms. The aromatic compounds have complete conjugation. All cyclic compounds with ten pi electrons will always be aromatic if they are planer. The aromatic compounds are more stable than the anti-aromatic.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




