
Pyrolysis of ethyl acetate gives:
A. \[C{H_3}COC{H_3}\]
B. \[C{H_2} = C{H_2}\]
C. \[C{H_2} = C = O\]
D. \[C{H_3} - CHO\]
Answer
233.1k+ views
Hint: Pyrolysis is the thermal decomposition of a substance at a particular temperature in an inert atmosphere, it involves a change of chemical composition. On decomposition of ester carboxylic acid and ethylene are formed.
Complete step by step answer:
Pyrolysis of ethyl acetate \[\left( {C{H_3}COOC{H_2}C{H_3}} \right)\] gives ethane and acetic acid.
\[\mathop {\left( {C{H_3}COOC{H_2}C{H_3}} \right)}\limits_{\left( {ethyl{\text{ }}acetate} \right)} \xrightarrow[{Pyrolysis}]{\Delta }\mathop {C{H_3}COOH}\limits_{\left( {acetic{\text{ }}acid} \right)} + \mathop {C{H_2} = {\text{ }}C{H_2}}\limits_{\left( {ethylene} \right)} \]
Ethyl acetate is heated in the presence of liquid nitrogen and glass wool.
Pyrolysis reaction is converting esters containing a $\beta - $hydrogen atom into the corresponding carboxylic acid and alkene. The reaction is a unimolecular elimination and operates in a syn-elimination. The mechanism involves a unimolecular six – centered transition state producing equimolar ethylene and acetic acid.
Mechanism of pyrolysis of ethyl acetate
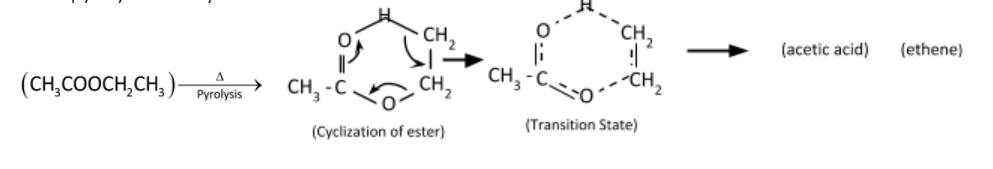
Therefore, the correct option is (D).
Note:
Ethyl acetate is a colorless liquid and is commonly known as ethyl ethanoate. Ethyl acetate is a polar aprotic solvent and it is a standard solvent. It is an important chemical compound. It is used especially for paints, varnishes, lacquers, cleaning and perfumes. The thermal decomposition of ethyl acetate follows a first order unimolecular reaction. The kinetic and thermodynamic parameters are obtained experimentally by applying the Arrhenius equation at a temperature range from $400^\circ $ to $600^\circ $C. The products from the pyrolysis of ethyl acetate were analyzed by gas chromatography.
Complete step by step answer:
Pyrolysis of ethyl acetate \[\left( {C{H_3}COOC{H_2}C{H_3}} \right)\] gives ethane and acetic acid.
\[\mathop {\left( {C{H_3}COOC{H_2}C{H_3}} \right)}\limits_{\left( {ethyl{\text{ }}acetate} \right)} \xrightarrow[{Pyrolysis}]{\Delta }\mathop {C{H_3}COOH}\limits_{\left( {acetic{\text{ }}acid} \right)} + \mathop {C{H_2} = {\text{ }}C{H_2}}\limits_{\left( {ethylene} \right)} \]
Ethyl acetate is heated in the presence of liquid nitrogen and glass wool.
Pyrolysis reaction is converting esters containing a $\beta - $hydrogen atom into the corresponding carboxylic acid and alkene. The reaction is a unimolecular elimination and operates in a syn-elimination. The mechanism involves a unimolecular six – centered transition state producing equimolar ethylene and acetic acid.
Mechanism of pyrolysis of ethyl acetate

Therefore, the correct option is (D).
Note:
Ethyl acetate is a colorless liquid and is commonly known as ethyl ethanoate. Ethyl acetate is a polar aprotic solvent and it is a standard solvent. It is an important chemical compound. It is used especially for paints, varnishes, lacquers, cleaning and perfumes. The thermal decomposition of ethyl acetate follows a first order unimolecular reaction. The kinetic and thermodynamic parameters are obtained experimentally by applying the Arrhenius equation at a temperature range from $400^\circ $ to $600^\circ $C. The products from the pyrolysis of ethyl acetate were analyzed by gas chromatography.
Recently Updated Pages
JEE Main 2023 April 6 Shift 1 Question Paper with Answer Key

JEE Main 2023 April 6 Shift 2 Question Paper with Answer Key

JEE Main 2023 (January 31 Evening Shift) Question Paper with Solutions [PDF]

JEE Main 2023 January 30 Shift 2 Question Paper with Answer Key

JEE Main 2023 January 25 Shift 1 Question Paper with Answer Key

JEE Main 2023 January 24 Shift 2 Question Paper with Answer Key

Trending doubts
JEE Main 2026: Session 2 Registration Open, City Intimation Slip, Exam Dates, Syllabus & Eligibility

JEE Main 2026 Application Login: Direct Link, Registration, Form Fill, and Steps

JEE Main Marking Scheme 2026- Paper-Wise Marks Distribution and Negative Marking Details

Understanding the Angle of Deviation in a Prism

Hybridisation in Chemistry – Concept, Types & Applications

How to Convert a Galvanometer into an Ammeter or Voltmeter

Other Pages
JEE Advanced Marks vs Ranks 2025: Understanding Category-wise Qualifying Marks and Previous Year Cut-offs

NCERT Solutions For Class 12 Chemistry Chapter 1 Solutions (2025-26)

Solutions Class 12 Chemistry Chapter 1 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 4 The d and f Block Elements (2025-26)

Biomolecules Class 12 Chemistry Chapter 10 CBSE Notes - 2025-26

NCERT Solutions For Class 12 Chemistry Chapter 10 Biomolecules (2025-26)




