
Pyrophosphorous acid has the molecular formula:
A. ${{H}_{3}}P{{O}_{3}}$
B. ${{H}_{3}}P{{O}_{4}}$
C. ${{H}_{4}}{{P}_{2}}{{O}_{5}}$
D. ${{H}_{4}}{{P}_{2}}{{O}_{7}}$
Answer
586.5k+ views
Hint: Think about the suffix ‘pyro-’ that is present before the phosphorous acid. Consider what it may indicate regarding the process while the preparation of the same. By using this you can easily find the correct option from the given options.
Complete step by step answer:
- Try to recall that pyrophosphorous acid is a glassy solid and is prepared by heating phosphorous acid.
- It is known to you that pyrophosphorous acid is a dibasic and it has one $P-O-P$ bond.
- Now, we will look at the structure of each of the acids given in the options and narrow down the answer.
- Option A:
${{H}_{3}}P{{O}_{3}}$ - This is the molecular formula of orthophosphorous acid or phosphonic acid. It is dibasic but does not contain any $P-O-P$ bond. So, this option is incorrect. The structure is:
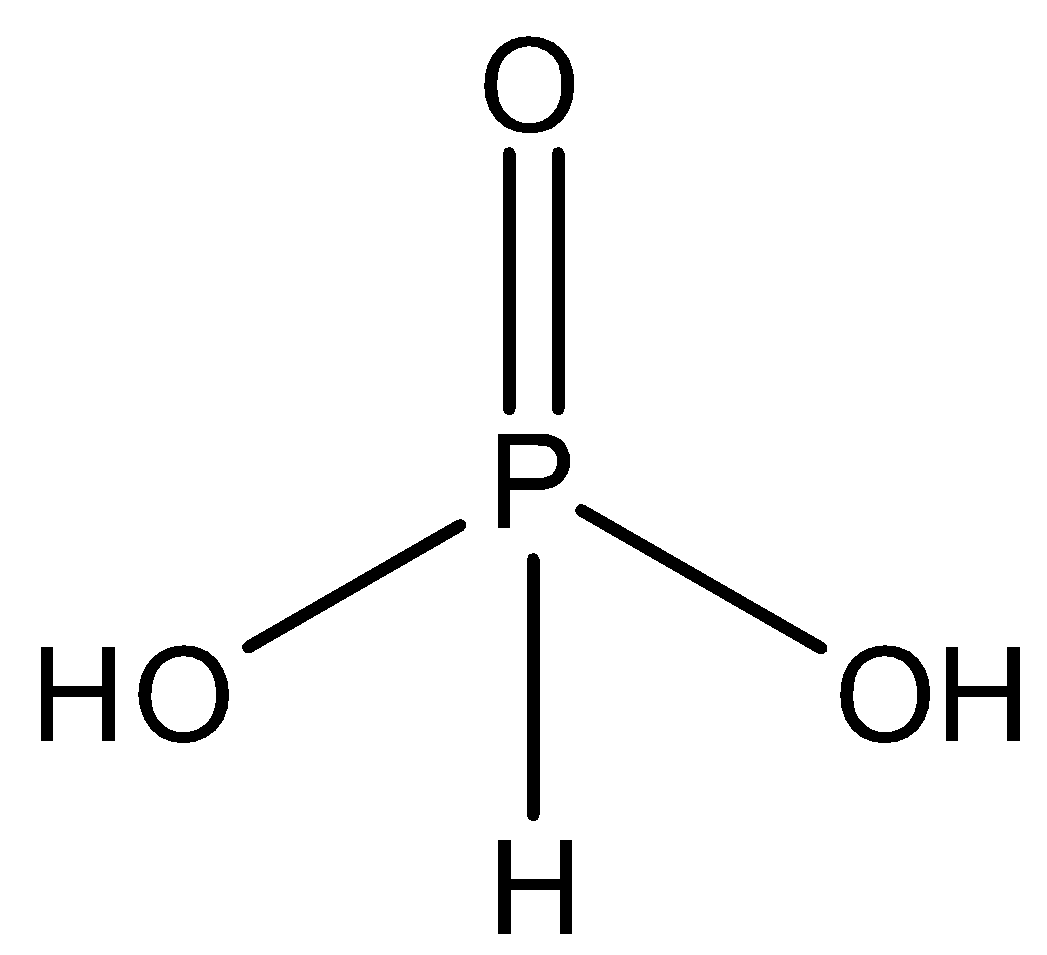
- Option B:
${{H}_{3}}P{{O}_{4}}$ - It is the molecular formula of orthophosphoric acid. It is tribasic or triprotic and does not contain any $P-O-P$ bond in its structure. So, this option is also not correct. The structure is:
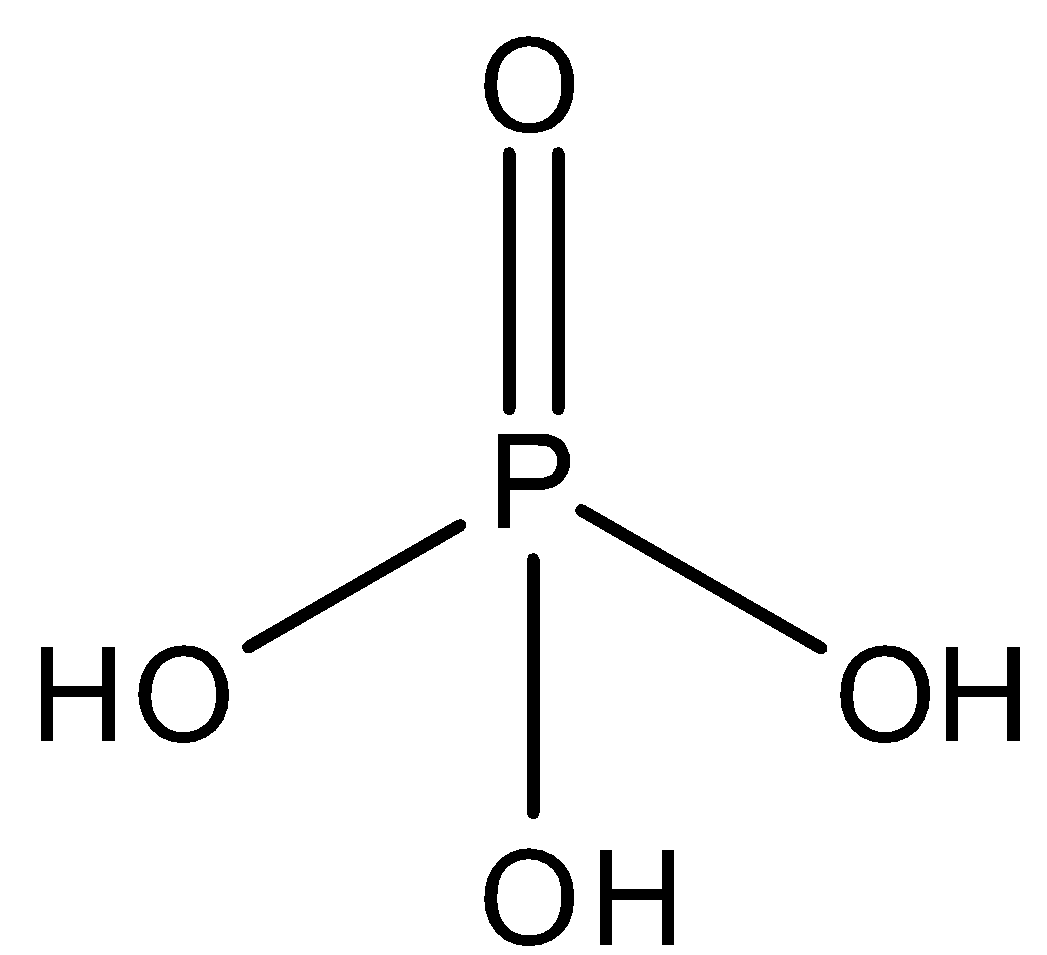
- Option C:
${{H}_{4}}{{P}_{2}}{{O}_{5}}$ - This acid is also dibasic and it has one $P-O-P$ bond in its structure. So, this is the molecular formula of pyrophosphorous acid and option C is the correct option. The structure is:
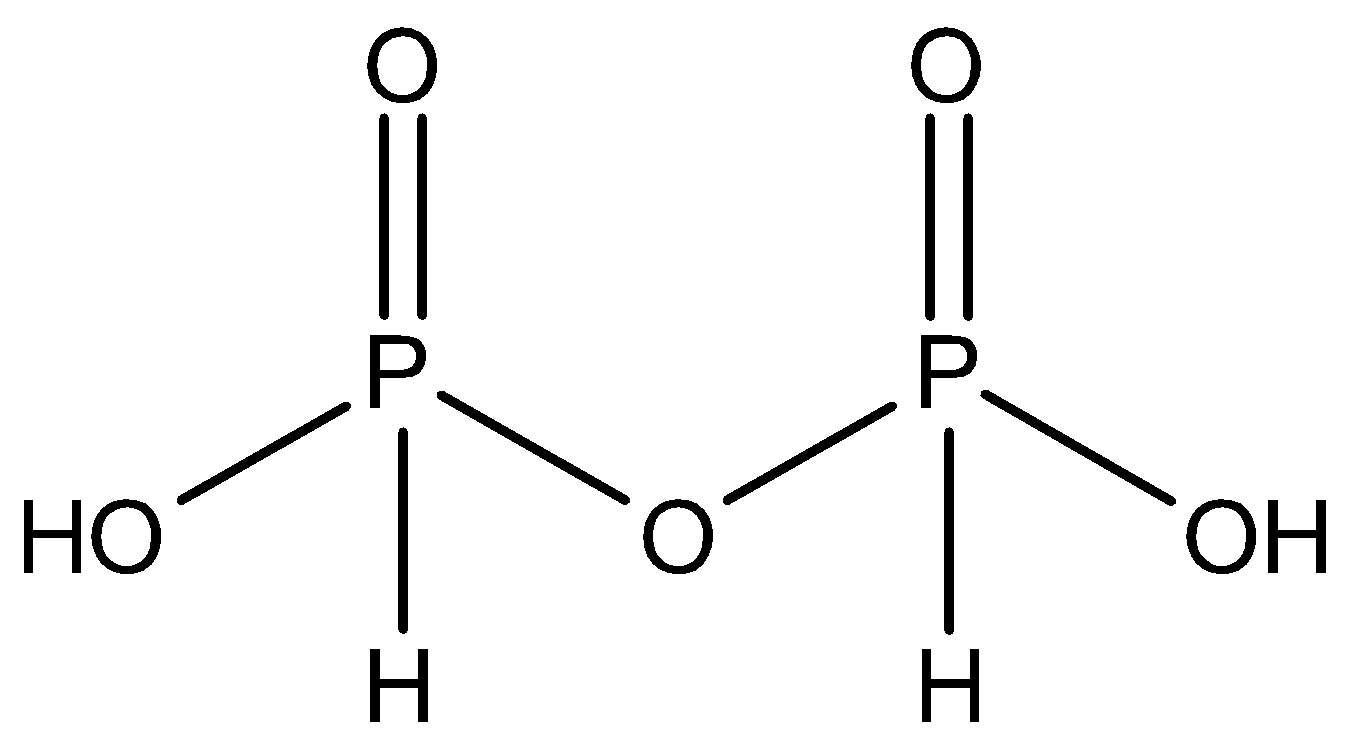
- Option D:
${{H}_{4}}{{P}_{2}}{{O}_{7}}$ - This is the molecular formula of pyrophosphoric acid. It is tetrabasic and has one $P-O-P$ bond in its structure. So, option D is also not correct.
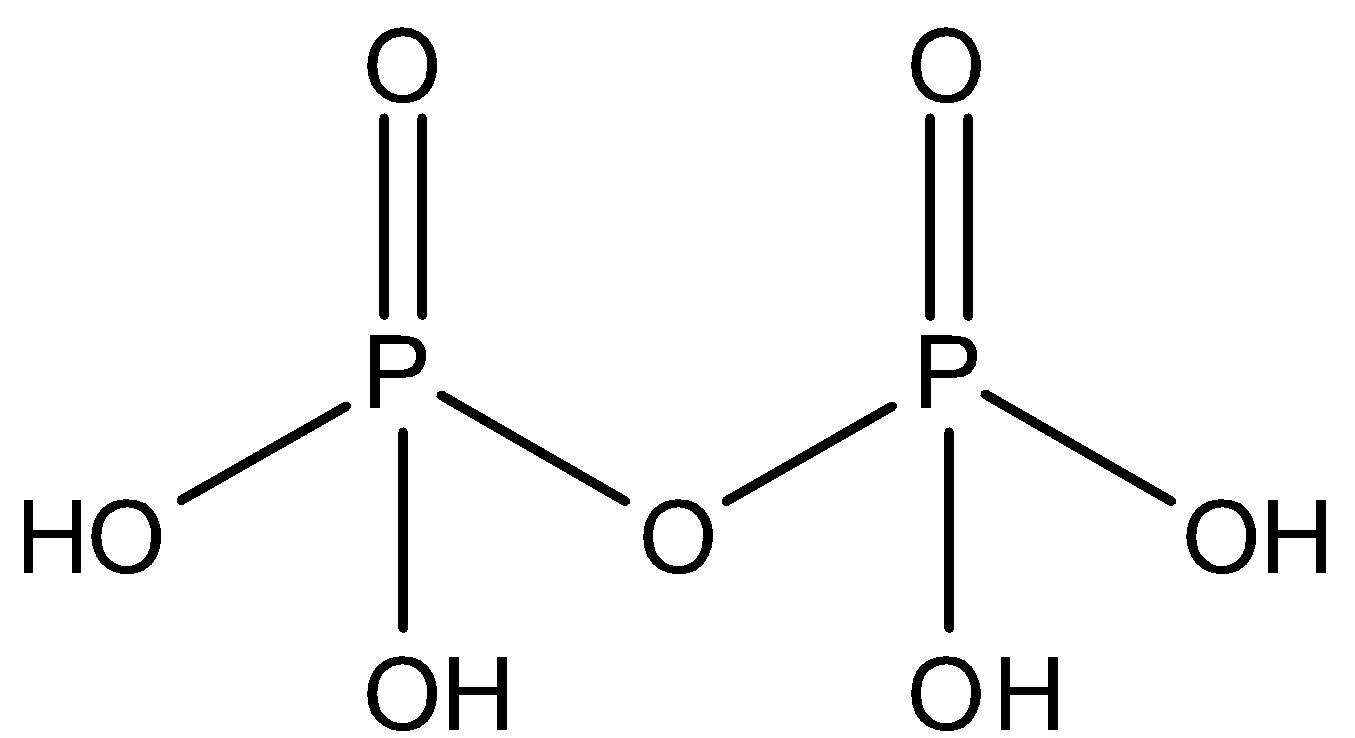
So, the correct answer is “Option C”.
Note: Remember that both pyrophosphorous acid and pyrophosphoric acid are formed by the dehydration of phosphorus and phosphoric acid respectively. When they are treated with heat, dehydration occurs and two phosphorus molecules are joined by one oxygen molecule.
Complete step by step answer:
- Try to recall that pyrophosphorous acid is a glassy solid and is prepared by heating phosphorous acid.
- It is known to you that pyrophosphorous acid is a dibasic and it has one $P-O-P$ bond.
- Now, we will look at the structure of each of the acids given in the options and narrow down the answer.
- Option A:
${{H}_{3}}P{{O}_{3}}$ - This is the molecular formula of orthophosphorous acid or phosphonic acid. It is dibasic but does not contain any $P-O-P$ bond. So, this option is incorrect. The structure is:

- Option B:
${{H}_{3}}P{{O}_{4}}$ - It is the molecular formula of orthophosphoric acid. It is tribasic or triprotic and does not contain any $P-O-P$ bond in its structure. So, this option is also not correct. The structure is:

- Option C:
${{H}_{4}}{{P}_{2}}{{O}_{5}}$ - This acid is also dibasic and it has one $P-O-P$ bond in its structure. So, this is the molecular formula of pyrophosphorous acid and option C is the correct option. The structure is:

- Option D:
${{H}_{4}}{{P}_{2}}{{O}_{7}}$ - This is the molecular formula of pyrophosphoric acid. It is tetrabasic and has one $P-O-P$ bond in its structure. So, option D is also not correct.

So, the correct answer is “Option C”.
Note: Remember that both pyrophosphorous acid and pyrophosphoric acid are formed by the dehydration of phosphorus and phosphoric acid respectively. When they are treated with heat, dehydration occurs and two phosphorus molecules are joined by one oxygen molecule.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

10 examples of friction in our daily life




