
(R)-$2$-iodobutane is treated with $NaI$ in acetone and allow to stand for a long time .the product eventually formed is:
A.(R)- $2$-iodobutane
B.(S)- $2$- iodobutane
C.$ \pm 2$-iodobutane
D.$( \pm ) - 1,2 - $iodobutane
Answer
572.1k+ views
Hint: The $S{N_2}$ reaction is a nucleophilic substitution reaction, in this a bond is broken and another is shaped synchronously. Two reacting species are kept inside the rate determining step of the reaction. The term '$S{N_2}$' stands for – Substitution Nucleophilic Bimolecular.
Complete Step by step solution:
${I^{ - 1}}$ is a good nucleophile as well as a very good leaving group. Therefore, if (R)-$2$ -iodobutane is controlled with $NaI$, then it’s repeated $S{N_2}$ reactions occur. As a final result, later a racemic aggregate of $( \pm ) - 2 - $ iodobutane is acquired.
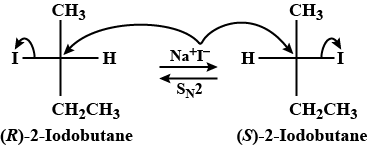
If we see the above explanation according to it, the correct answer is $ \pm 2$-iodobutane .
So, the correct answer is option C.
Additional information:
The answer proceeds through a bottom attack by the nucleophile on the substrate. The nucleophile methods the given substrate at an altitude of $180$ degree to the carbon-leaving institution bond or to the carbon leaving group. The carbon-nucleophile bond forms and carbon-leaving organization bond breaks simultaneously through a transition nation.
Now, the leaving organization is driven out of the transition state on the other side of the carbon-nucleophile bond, forming the specified product. It is vital to note that the product is shaped with an inversion of the tetrahedral geometry on the atom within the center.
Note: The $S{N_2}$ reaction is a good example of stereospecific reactions. In this one there are in which one of a kind stereoisomers react to provide different stereoisomers of the product. Also, $S{N_2}$ response is the highest common instance of Walden inversion, in which an uneven carbon atom(that is an odd number of carbon) undergoes inversion of configurations.
Complete Step by step solution:
${I^{ - 1}}$ is a good nucleophile as well as a very good leaving group. Therefore, if (R)-$2$ -iodobutane is controlled with $NaI$, then it’s repeated $S{N_2}$ reactions occur. As a final result, later a racemic aggregate of $( \pm ) - 2 - $ iodobutane is acquired.

If we see the above explanation according to it, the correct answer is $ \pm 2$-iodobutane .
So, the correct answer is option C.
Additional information:
The answer proceeds through a bottom attack by the nucleophile on the substrate. The nucleophile methods the given substrate at an altitude of $180$ degree to the carbon-leaving institution bond or to the carbon leaving group. The carbon-nucleophile bond forms and carbon-leaving organization bond breaks simultaneously through a transition nation.
Now, the leaving organization is driven out of the transition state on the other side of the carbon-nucleophile bond, forming the specified product. It is vital to note that the product is shaped with an inversion of the tetrahedral geometry on the atom within the center.
Note: The $S{N_2}$ reaction is a good example of stereospecific reactions. In this one there are in which one of a kind stereoisomers react to provide different stereoisomers of the product. Also, $S{N_2}$ response is the highest common instance of Walden inversion, in which an uneven carbon atom(that is an odd number of carbon) undergoes inversion of configurations.
Recently Updated Pages
Master Class 11 Computer Science: Engaging Questions & Answers for Success

Master Class 11 Business Studies: Engaging Questions & Answers for Success

Master Class 11 Economics: Engaging Questions & Answers for Success

Master Class 11 English: Engaging Questions & Answers for Success

Master Class 11 Maths: Engaging Questions & Answers for Success

Master Class 11 Biology: Engaging Questions & Answers for Success

Trending doubts
One Metric ton is equal to kg A 10000 B 1000 C 100 class 11 physics CBSE

There are 720 permutations of the digits 1 2 3 4 5 class 11 maths CBSE

Discuss the various forms of bacteria class 11 biology CBSE

Draw a diagram of a plant cell and label at least eight class 11 biology CBSE

State the laws of reflection of light

Explain zero factorial class 11 maths CBSE




