
What is the rate determining step in an ${{\text{S}}_{\text{N}}}\text{2}$ reaction?
Answer
525.3k+ views
Hint: The ${{\text{S}}_{\text{N}}}\text{2}$ reaction stands for Substitution Nucleophilic Bimolecular reaction. A nucleophile attacks the substrate and leads to the simultaneous breaking and formation of bonds between the carbon-leaving group and carbon-nucleophile respectively. The rate is determined by the reaction between substrate and nucleophile.
Complete answer:
Many chemical reactions occur in more than one step and follow a particular reaction mechanism. The rate determining step in any chemical reaction is the slowest step that determines the speed or the rate at which the reaction will proceed. The rate equation of such multistep reactions involves the rate constant and concentration terms of the rate determining step.
Before discussing the rate determining step of an ${{\text{S}}_{\text{N}}}\text{2}$ reaction, let us take a brief look at its reaction mechanism.
The ${{\text{S}}_{\text{N}}}\text{2}$ reaction is a bimolecular nucleophilic substitution reaction. Now, the nucleophile is those groups that have electron-rich atoms capable of donating their electron pairs to an electrophile. The alkyl halides generally undergo an ${{\text{S}}_{\text{N}}}\text{2}$ reaction.
The nucleophile approaches the electron-deficient carbon atom (substrate) from the back. The bond formation between nucleophile and carbon takes place simultaneously with the breaking of the bond between carbon and a halogen atom.
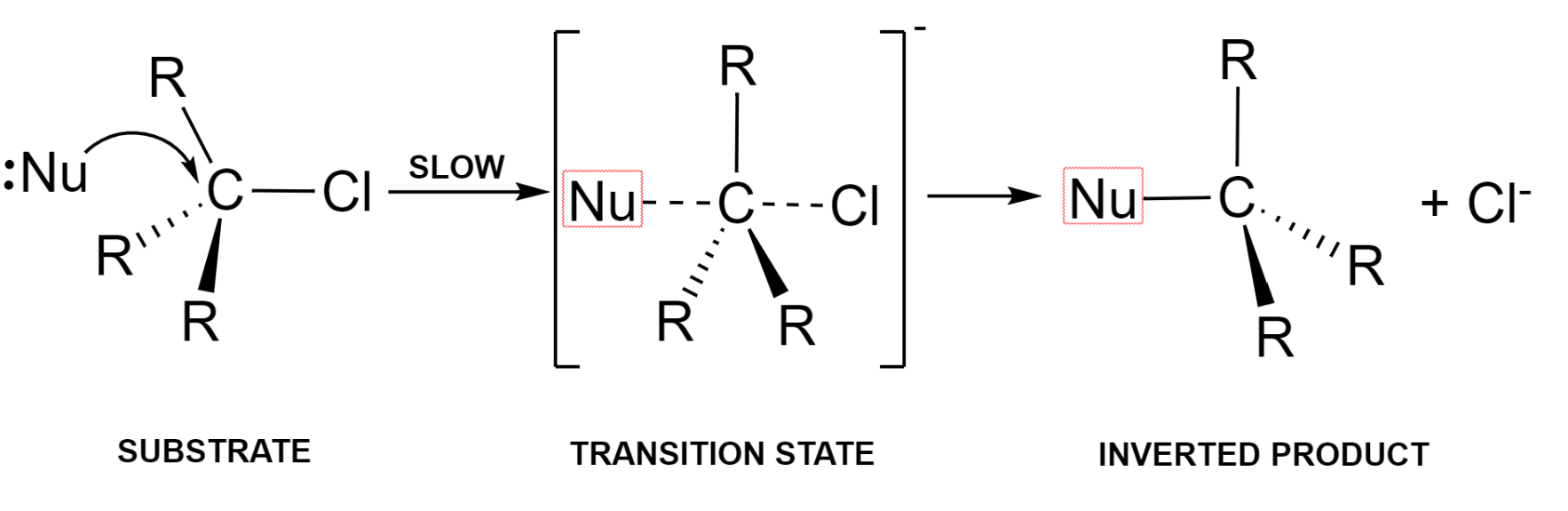
This is known as the transition state which later on pushes out the halide ion as a leaving group and forms the required product with an inversion of geometry at the centre.
Here, the rate determining step is the step where the formation of transition state takes place, that is the interaction between the nucleophile and the organic substrate.
Note:
The ${{\text{S}}_{\text{N}}}\text{2}$ reaction is said to be a single-step process involving a single transition state formation but a transition state is a very short-lived species that cannot be observed directly. The 2 in ${{\text{S}}_{\text{N}}}\text{2}$ stands for bimolecular as the rate of the reaction depends on both the nucleophile and the substrate group.
Complete answer:
Many chemical reactions occur in more than one step and follow a particular reaction mechanism. The rate determining step in any chemical reaction is the slowest step that determines the speed or the rate at which the reaction will proceed. The rate equation of such multistep reactions involves the rate constant and concentration terms of the rate determining step.
Before discussing the rate determining step of an ${{\text{S}}_{\text{N}}}\text{2}$ reaction, let us take a brief look at its reaction mechanism.
The ${{\text{S}}_{\text{N}}}\text{2}$ reaction is a bimolecular nucleophilic substitution reaction. Now, the nucleophile is those groups that have electron-rich atoms capable of donating their electron pairs to an electrophile. The alkyl halides generally undergo an ${{\text{S}}_{\text{N}}}\text{2}$ reaction.
The nucleophile approaches the electron-deficient carbon atom (substrate) from the back. The bond formation between nucleophile and carbon takes place simultaneously with the breaking of the bond between carbon and a halogen atom.

This is known as the transition state which later on pushes out the halide ion as a leaving group and forms the required product with an inversion of geometry at the centre.
Here, the rate determining step is the step where the formation of transition state takes place, that is the interaction between the nucleophile and the organic substrate.
Note:
The ${{\text{S}}_{\text{N}}}\text{2}$ reaction is said to be a single-step process involving a single transition state formation but a transition state is a very short-lived species that cannot be observed directly. The 2 in ${{\text{S}}_{\text{N}}}\text{2}$ stands for bimolecular as the rate of the reaction depends on both the nucleophile and the substrate group.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Why cannot DNA pass through cell membranes class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE

In a human foetus the limbs and digits develop after class 12 biology CBSE

AABbCc genotype forms how many types of gametes a 4 class 12 biology CBSE

Differentiate between homogeneous and heterogeneous class 12 chemistry CBSE

The correct structure of ethylenediaminetetraacetic class 12 chemistry CBSE




