
Reaction of ${\text{RCON}}{{\text{H}}_{\text{2}}}$ with a mixture of ${\text{B}}{{\text{r}}_{\text{2}}}$ and KOH gives ${\text{RN}}{{\text{H}}_{\text{2}}}$ as the main product. The intermediate involved in the reaction is:
(A)
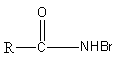
B.${\text{R}} - {\text{NHBr}}$
(C)
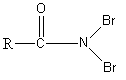
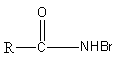

Answer
568.8k+ views
Hint:: The amide can be converted into amine by reacting the amide with bromine and sodium hydroxide. The reaction of conversion of primary amide into amine in presence of bromine with potassium hydroxide is known as Hoffmann bromamide reaction. To determine the answer we should know the mechanism of the reaction.
Complete step by step solution:Hoffmann bromamide reaction is used for the preparation of primary amine from amide. The reaction is shown as follows:
${\text{RCON}}{{\text{H}}_{\text{2}}}\,{\text{ + }}\,{\text{Br}}\,{\text{ + }}\,{\text{KOH}}\,\, \to \,\,{\text{RN}}{{\text{H}}_2}{\text{ + }}\,{\text{2}}\,{\text{NaBr}}\,{\text{ + }}\,{{\text{K}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\,{\text{ + }}\,{\text{2}}{{\text{H}}_{\text{2}}}{\text{O}}$
The mechanism of the reaction is as follows:
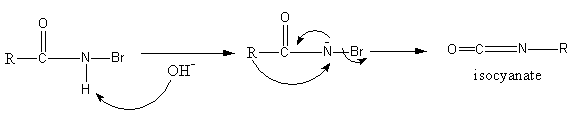
Initially, the base sodium hydroxide abstract proton from amide to generate an anion of amide. The anion of amide now attacks on bromine, so an N-bromoamide generates.
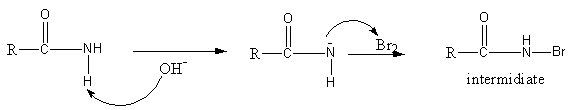
Again the base attacks on N-bromoamide so an anion of bromoamide forms. Then the methyl group attached with the carbonyl group shifts to the nitrogen atom and bromide ion leaves forming an isocyanate structure.
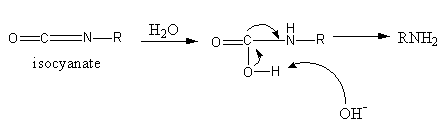
Nucleophilic attack of water on isocyanate takes place which loses the carbon dioxide and forms a structure in which nitrogen has a negative charge and one hydrogen and one methyl group. Then the protonation of this nitrogen takes place which gives amine.
So, the intermediate involved in the reaction is,
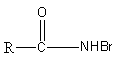
Therefore, option (A) is correct.
Note: The Hoffmann bromamide reaction is used for the preparation of primary amine only. One important structure form during the reaction is isocyanate. Lossen rearrangement, Beckmann rearrangement and Hofmann reaction, all are used for the preparation of primary amine. Hoffmann bromamide reaction and Lossen rearrangement are used for the preparation of primary amine only via the formation of isocyanate. Beckmann rearrangement gives amides from oximes.
Complete step by step solution:Hoffmann bromamide reaction is used for the preparation of primary amine from amide. The reaction is shown as follows:
${\text{RCON}}{{\text{H}}_{\text{2}}}\,{\text{ + }}\,{\text{Br}}\,{\text{ + }}\,{\text{KOH}}\,\, \to \,\,{\text{RN}}{{\text{H}}_2}{\text{ + }}\,{\text{2}}\,{\text{NaBr}}\,{\text{ + }}\,{{\text{K}}_{\text{2}}}{\text{C}}{{\text{O}}_{\text{3}}}\,{\text{ + }}\,{\text{2}}{{\text{H}}_{\text{2}}}{\text{O}}$
The mechanism of the reaction is as follows:
Initially, the base sodium hydroxide abstract proton from amide to generate an anion of amide. The anion of amide now attacks on bromine, so an N-bromoamide generates.

Again the base attacks on N-bromoamide so an anion of bromoamide forms. Then the methyl group attached with the carbonyl group shifts to the nitrogen atom and bromide ion leaves forming an isocyanate structure.

Nucleophilic attack of water on isocyanate takes place which loses the carbon dioxide and forms a structure in which nitrogen has a negative charge and one hydrogen and one methyl group. Then the protonation of this nitrogen takes place which gives amine.

So, the intermediate involved in the reaction is,
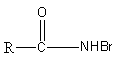
Therefore, option (A) is correct.
Note: The Hoffmann bromamide reaction is used for the preparation of primary amine only. One important structure form during the reaction is isocyanate. Lossen rearrangement, Beckmann rearrangement and Hofmann reaction, all are used for the preparation of primary amine. Hoffmann bromamide reaction and Lossen rearrangement are used for the preparation of primary amine only via the formation of isocyanate. Beckmann rearrangement gives amides from oximes.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

What is a transformer Explain the principle construction class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE




