
Reduction of nitrobenzene in the presence of Zn \[N{{H}_{4}}Cl\] gives:
A) Hydrazobenzene
B) Aniline
C) Azobenzene
D) N-phenylhydroxylamine
Answer
598.5k+ views
Hint: Ammonium salts, such as ammonium formate and acetate, can also be used as a promoter for zinc reduction. Promoters, like ammonium chloride, serve as electrolytes and complex forming agents to enable zinc reduction of nitrobenzene to proceed at near ambient temperatures.
Complete answer:
For reduction of a nitro compound into an amine, it requires six units of reducing agent.
\[R-N{{O}_{2}}+6{{H}^{+}}+6{{e}^{-}}\to RN{{H}_{2}}+2{{H}_{2}}O\]
The whole reaction doesn’t occur in a single step, instead it requires multiple steps with forming a chain of intermediates, which with strong reducing agents in acidic solution have a small transient existence. Intermediates formed by adding two units of reducing agents to \[RN{{O}_{2}}\] are nitroso compounds, \[RN=O\]and N-substituted hydroxylamines \[RNHOH\].
\[RN{{O}_{2}}\xrightarrow[-{{H}_{2}}O]{2[H]}RN=O\xrightarrow{2[H]}RNHOH\xrightarrow[-{{H}_{2}}O]{2[H]}RN{{H}_{2}}\]
The n- substituted hydroxylamine can be directly obtained from the respective nitro compounds with Zn and \[N{{H}_{4}}Cl\].
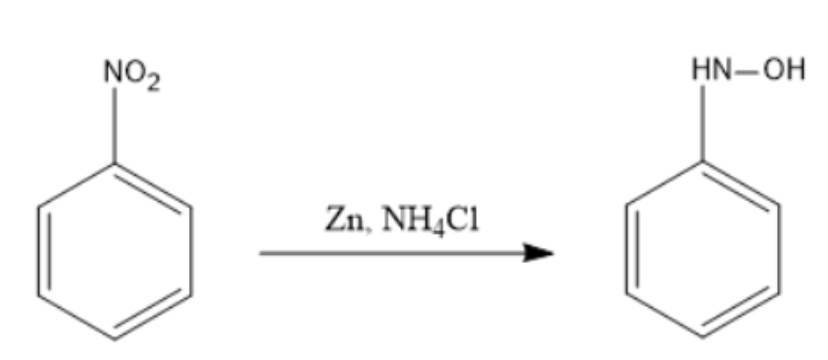
Reduction of nitrobenzene with the help of Zn is an electrolytic reaction done in the aqueous phase, where an electrolyte is required to serve as the promoter. Generally, ammonium chloride \[N{{H}_{4}}Cl\] is used as a promoter and the reaction is carried out at a temperature of 50℃– 65℃.
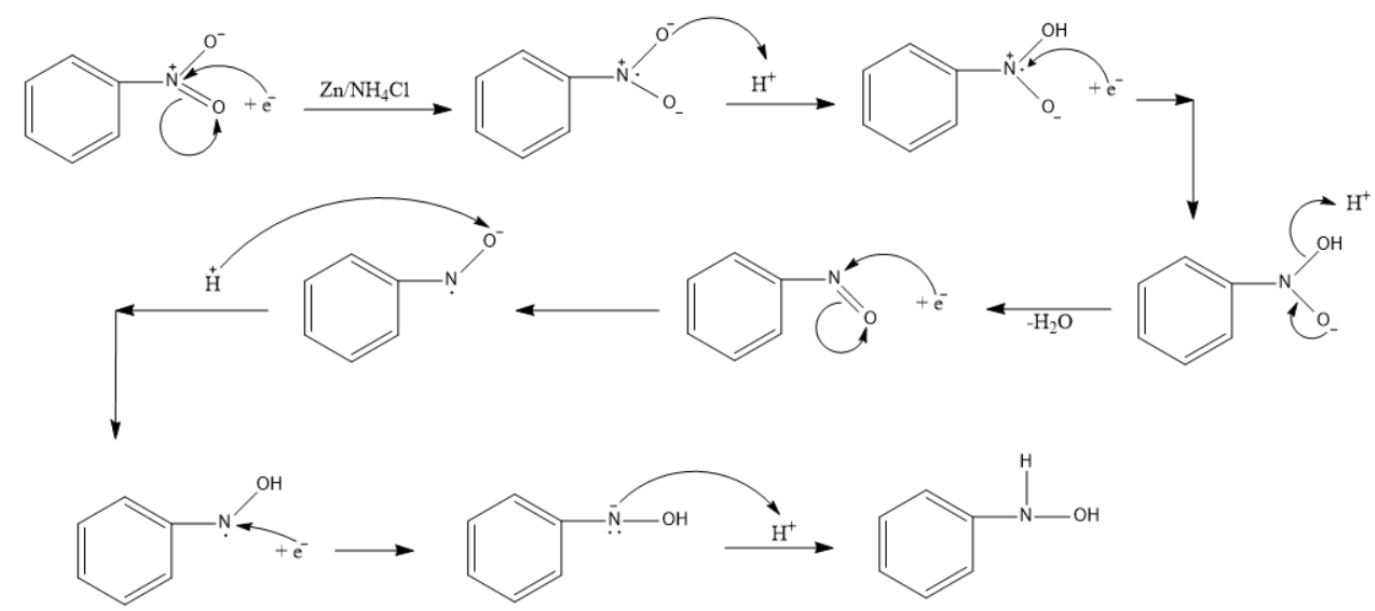
The exact mechanism for the formation of N-substituted hydroxylamine can be seen below.
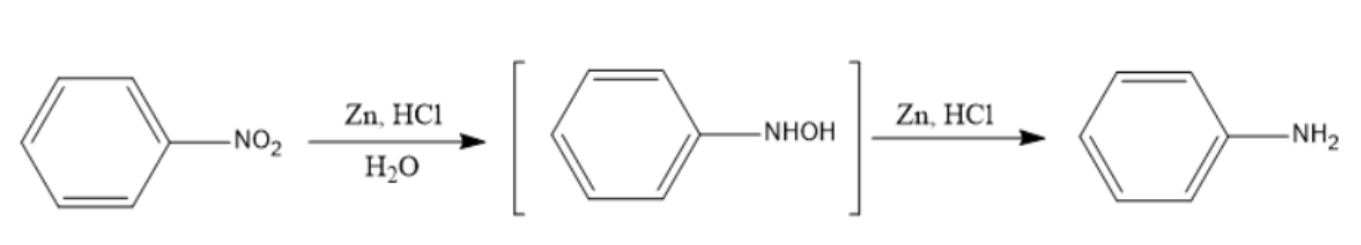
Note: Under mildly alkaline conditions, the reduction is terminated at the formation of N-substituted hydroxylamine. But under acidic conditions, the reaction proceeds further to form aniline as seen in the picture below. In the second branch we have used Zn and HCl instead of Zn and \[N{{H}_{4}}Cl\].
Complete answer:
For reduction of a nitro compound into an amine, it requires six units of reducing agent.
\[R-N{{O}_{2}}+6{{H}^{+}}+6{{e}^{-}}\to RN{{H}_{2}}+2{{H}_{2}}O\]
The whole reaction doesn’t occur in a single step, instead it requires multiple steps with forming a chain of intermediates, which with strong reducing agents in acidic solution have a small transient existence. Intermediates formed by adding two units of reducing agents to \[RN{{O}_{2}}\] are nitroso compounds, \[RN=O\]and N-substituted hydroxylamines \[RNHOH\].
\[RN{{O}_{2}}\xrightarrow[-{{H}_{2}}O]{2[H]}RN=O\xrightarrow{2[H]}RNHOH\xrightarrow[-{{H}_{2}}O]{2[H]}RN{{H}_{2}}\]
The n- substituted hydroxylamine can be directly obtained from the respective nitro compounds with Zn and \[N{{H}_{4}}Cl\].

Reduction of nitrobenzene with the help of Zn is an electrolytic reaction done in the aqueous phase, where an electrolyte is required to serve as the promoter. Generally, ammonium chloride \[N{{H}_{4}}Cl\] is used as a promoter and the reaction is carried out at a temperature of 50℃– 65℃.
The exact mechanism for the formation of N-substituted hydroxylamine can be seen below.

Note: Under mildly alkaline conditions, the reduction is terminated at the formation of N-substituted hydroxylamine. But under acidic conditions, the reaction proceeds further to form aniline as seen in the picture below. In the second branch we have used Zn and HCl instead of Zn and \[N{{H}_{4}}Cl\].

Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




