
How reduction of the following takes place:
i.Reduction of acetaldehyde
ii.Reduction of ester
Answer
514.5k+ views
Hint: When a loss in oxygen atom i.e., an electronegative element or a gain in hydrogen atom i.e., electropositive element is observed within a compound during an organic reaction, then it is said to be a reduction reaction. The reduction of a compound leads to net decrease in the electronegativity of the compound.
Complete answer: The reduction of the given compounds takes place as follows:
(a) Reduction of acetaldehyde:
Step-1: Reaction of acetaldehyde with $NaB{H_4}$ to form the respective sodium derivative along with the removal of $B{H_3}$. The reaction takes place as follows:
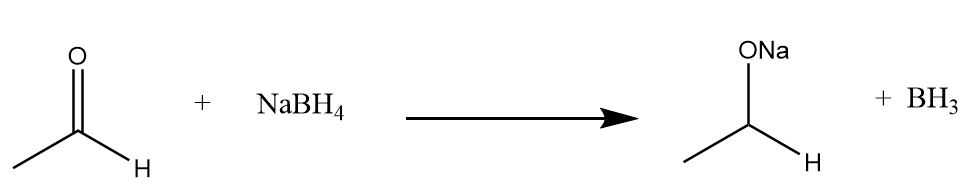
Step-2: Protonation of the given compound occurs in the presence of water molecules to form primary alcohol. The reaction proceeds as follows:
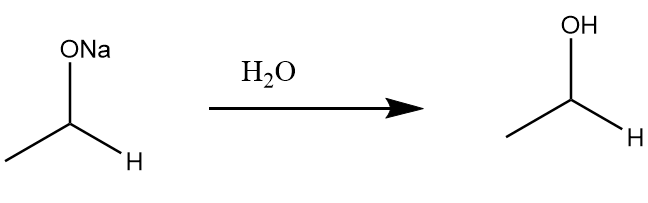
Hence, formation of ethanol takes place on the reduction of acetaldehyde.
(b) Reduction of ester:
Step-1: The hydrogen ion from $LiAl{H_4}$ attacks the carbonyl centre of the ester and formation of respective aldehyde takes place along with the removal of $OR$ group. The reaction proceeds as follows:
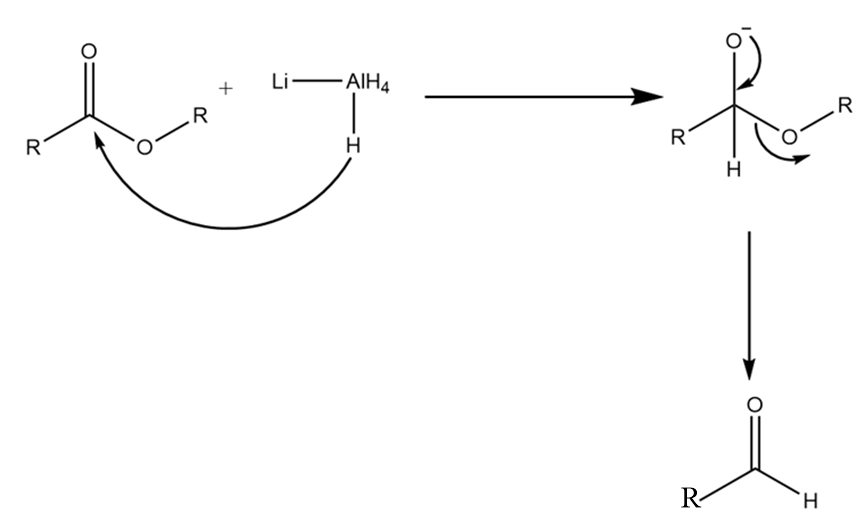
Step-2: The hydrogen ion of $LiAl{H_4}$ again attacks the carbonyl centre of aldehyde and the compound is reduced to its respective alcohol. The reaction proceeds as follows:
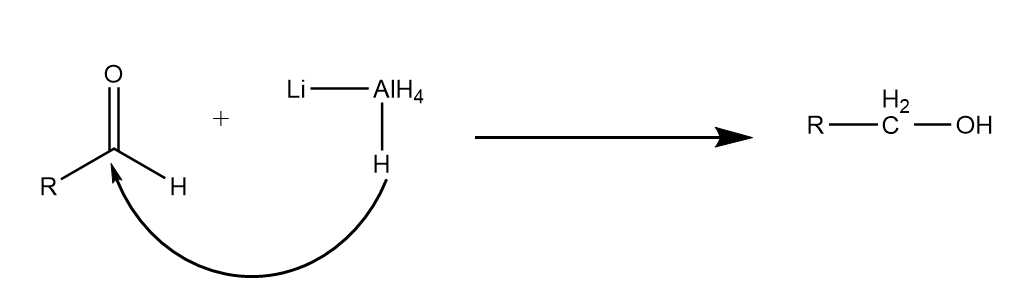
Hence, on reduction of ester the formation of primary alcohol takes place.
Note:
It is important to note that $NaB{H_4}$ is a mild reducing agent and can only be used in the reduction reaction of aldehydes and ketones. It cannot be used for the reduction of carboxylic acids and esters. Only strong reducing agents like $LiAl{H_4}$ can be used for the reduction of esters and acids.
Complete answer: The reduction of the given compounds takes place as follows:
(a) Reduction of acetaldehyde:
Step-1: Reaction of acetaldehyde with $NaB{H_4}$ to form the respective sodium derivative along with the removal of $B{H_3}$. The reaction takes place as follows:
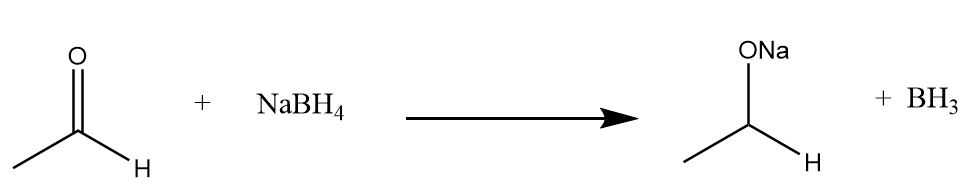
Step-2: Protonation of the given compound occurs in the presence of water molecules to form primary alcohol. The reaction proceeds as follows:

Hence, formation of ethanol takes place on the reduction of acetaldehyde.
(b) Reduction of ester:
Step-1: The hydrogen ion from $LiAl{H_4}$ attacks the carbonyl centre of the ester and formation of respective aldehyde takes place along with the removal of $OR$ group. The reaction proceeds as follows:

Step-2: The hydrogen ion of $LiAl{H_4}$ again attacks the carbonyl centre of aldehyde and the compound is reduced to its respective alcohol. The reaction proceeds as follows:

Hence, on reduction of ester the formation of primary alcohol takes place.
Note:
It is important to note that $NaB{H_4}$ is a mild reducing agent and can only be used in the reduction reaction of aldehydes and ketones. It cannot be used for the reduction of carboxylic acids and esters. Only strong reducing agents like $LiAl{H_4}$ can be used for the reduction of esters and acids.
Recently Updated Pages
Master Class 12 Economics: Engaging Questions & Answers for Success

Master Class 12 Physics: Engaging Questions & Answers for Success

Master Class 12 English: Engaging Questions & Answers for Success

Master Class 12 Social Science: Engaging Questions & Answers for Success

Master Class 12 Maths: Engaging Questions & Answers for Success

Master Class 12 Business Studies: Engaging Questions & Answers for Success

Trending doubts
Which are the Top 10 Largest Countries of the World?

What are the major means of transport Explain each class 12 social science CBSE

Draw a labelled sketch of the human eye class 12 physics CBSE

Why cannot DNA pass through cell membranes class 12 biology CBSE

Differentiate between insitu conservation and exsitu class 12 biology CBSE

Draw a neat and well labeled diagram of TS of ovary class 12 biology CBSE




